Background:
The impact of previous radiotherapy on free flap outcome is still a subject of debate. Clinical investigations have come to divergent conclusions and the true effect of radiotherapy (XRT) on flap survival is not definitely known. Most studies investigating the factor often have their methodological limitations such as lack of statistical power as a consequence of the overall low failure rates together with few irradiated cases. This study will attempt to address the question whether previous radiotherapy is associated with a significantly higher incidence of flap failure or not.
Methods:
A systematic review and meta-analysis were conducted in concordance with the PRISMA protocol using the PubMed database. Fixed-effect and random-effect models were applied to obtain the odds ratio of total flap failure and partial flap failure between radiation and nonradiation groups. Statistical heterogeneity and publication bias were assessed and forest plots and funnel plots were constructed for graphic illustration.
Results:
A total of 43 studies were included for qualitative and quantitative analysis involving 18,776 flaps in 17,532 patients. Patients with preoperative XRT were significantly associated with an increased risk for total (odds ratio fixed = 1.675, 95% confidence interval [CI] = 1.405–1.996, P < 0.001) and partial free flap failure (odds ratio fixed = 2.161, 95% CI = 1.472–2.172, P < 0.001).
Conclusion:
The study suggests that preoperative radiotherapy is associated with an increased risk for total and partial free flap failure. Further studies are needed to investigate the effect of total XRT dose and time after radiation on free flap outcome.
Takeaways
Question: This study will attempt to address the question whether previous radiotherapy is associated with a significantly higher incidence of free flap failure or not.
Findings: A systematic review and meta-analysis were conducted in concordance with the PRISMA protocol using the PubMed database. The study suggests that preoperative radiotherapy is associated with an increased risk for total and partial free flap failure.
Meaning: Preoperative radiotherapy is associated with an increased risk for total and partial free flap failure and further outcome studies are needed, especially to investigate the effect of total XRT dose and time after radiation on free flap outcome.
INTRODUCTION
The benefits of radiotherapy for cancer have been well documented. However, the use of radiation therapy to treat cancer inevitably involves exposure of healthy tissue and the benefits can be outweighed by radiation-induced damage to neighboring healthy tissue as a result of either direct exposure to radiation or the so-called bystander effect, which refers to biological effects in nonirradiated cells caused by signals from irradiated cells.1,2 The pathological process of radiation injury begins immediately after radiation exposure, but the clinical and histological features may not become apparent for weeks, months, or even years after treatment.2
Radiation Injury in Microsurgery: Is There a Prothrombotic State?
Radiation therapy will also have adverse effects on small blood vessels in microsurgery. Histologic observations of irradiated vessels displayed more abnormalities, which included diminished smooth muscle density, endothelial cell dehiscence, and vessel wall fibrosis. There was a statistically significantly higher incidence of blood vessel lesions with fibrin deposition and microthrombi than in control vessels. Consequently, authors concluded that microvascular surgery in irradiated vessels carries with it a higher risk for thrombosis due to preexisting vessel wall damage.3 Several studies have shown that radiation negatively affects the vascular endothelium with enhanced proadhesive and prothrombotic properties with abnormal coagulation and fibrinolysis.4,5
Studies suggest that radiation may lead to a prothrombotic response. In one study, paired biopsies from radiated recipient veins and nonradiated flap veins were simultaneously harvested during free flap reconstruction to analyze differential gene expression in a large number of genes involved in inflammation and coagulation.6 By comparing radiated recipient veins with nonradiated veins from autologous tissue transfer in the same patient, Halle et al were able to eliminate interindividual differences and study the sole effect of radiation on gene expression. The results of the study indicated endothelial activation in radiated veins illustrated by an acute inflammatory response with increased cytokine and leukocyte adhesion molecule expression. The activated endothelium has thrombogenic properties, thereby promoting leukocyte or platelet endothelial adherence and thrombus formation. Moreover, a sustained increase in PAI-1, which is the main inhibitor of the fibrinolytic system, was detected. Consequently, it can be hypothesized that a prothrombotic state results through gene activation of plasminogen activator inhibitor.
Another study by the same group compared radiated and nonradiated arteries using the same method to retrieve samples. The results suggested sustained inflammation due to NF-κB activation in human radiated arteries leading to prothrombotic properties and proneness to atherosclerosis of the activated endothelium.7
Stone et al2 also concluded that radiotherapy is associated with activation of a range of cellular signaling pathways leading to expression of inflammatory cytokines with subsequent activation of the coagulation cascade and induction of vascular damage.
Schultz-Hector and Trott8 noted that in addition to proinflammatory responses, evidence points to prothrombotic effects of radiation because an increased deposit or release of von Willebrand factor was observed after irradiation of endothelial cells in vitro.
It has also been reported that radiation-induced endothelial injury could lead to a deficit of constitutive nitric oxide synthesis, which can promote platelet aggregation and thrombus formation.9 This is of particular interest since nitric oxide inhibitors have been shown to promote thrombosis in microvascular anastomosis.10
In theory, these biological effects may decrease viability of free tissue transfer with increased failure rates. Thus, questions have arisen regarding the effect of radiation in microvascular free tissue transfer and the effect on free flap failure.
A Subject to Debate
The impact of preoperative external radiotherapy on the outcome of free tissue transfers has been a subject of much interest. Although experimental work suggests lower patency rates of anastomoses performed on irradiated vessels,11,12 clinical studies have not been able to unanimously replicate these findings.
Numerous clinical investigations have come to divergent conclusions and the true effect of radiotherapy (XRT) on flap survival is not definitely known. Several authors showed that preoperative XRT may increase the flap failure rate.13–23 In contrast, some other studies concluded that XRT before free tissue transfer does not significantly increase flap loss.24–55 Controversy remains as to the exact effect of XRT on the flap failure rate. Although an increased risk for local complications in previously irradiated patients is widely acknowledged, a significant statistical correlation is not often found between free flap failure and preoperative radiation although most reports analyzing risk factors for flap failure often have their methodological limitations such as lack of statistical power as a consequence of the overall low failure rates together with few irradiated cases. To deal with the controversy, this review with meta-analysis was performed. This study will attempt to address the question whether previous radiotherapy is associated with a significantly higher incidence of flap failure or not.
METHODS
A comprehensive search strategy was developed to capture all relevant articles related to the review question whether prior radiation therapy is associated with an increased risk for flap failure in free tissue transfer or not. The literature was reviewed using both computerized and manual search methods. Initially, an electronic literature review was conducted using PubMed database without language or date restrictions. Two initial searches were applied using the following search terms:
SEARCH 1: radiation OR radiotherapy AND (“flap failure” OR “flap loss”)
SEARCH 2: “risk factors” AND “flap failure”
The latest search was conducted on December 15, 2020. The systematic search was performed using the Preferred Reporting Items for Systematic Reviews and Meta-analyses literature selection process (PRISMA).56 The search results were merged and deduplicated before screening. Titles and abstracts were then screened to eliminate irrelevant studies. Full texts were retrieved for the remaining studies that had passed the first level of screening and were selected using inclusion criteria defined before the search. Inclusion criteria required each study to clearly outline the total number of patients, the explicit statement of numbers in radiation and nonradiation groups, the microsurgical anastomosis in the radiation group in the radiated field and the number of flaps loss or flap failures in each group. Criteria of exclusion were case reports, animal studies, reviews, and articles not published in the English language. In addition, reference articles were screened manually to retrieve relevant studies. A manual review of references from review articles was also performed to identify additional studies. The identified studies were included for qualitative and quantitative synthesis. The data collected included the following information: flap loss/flap failure and partial flap loss in radiated and nonradiated patients and the area of reconstruction. If available, radiation dose and timing of reconstruction postradiation were additionally recorded.
RESULTS
Characteristics of the Studies
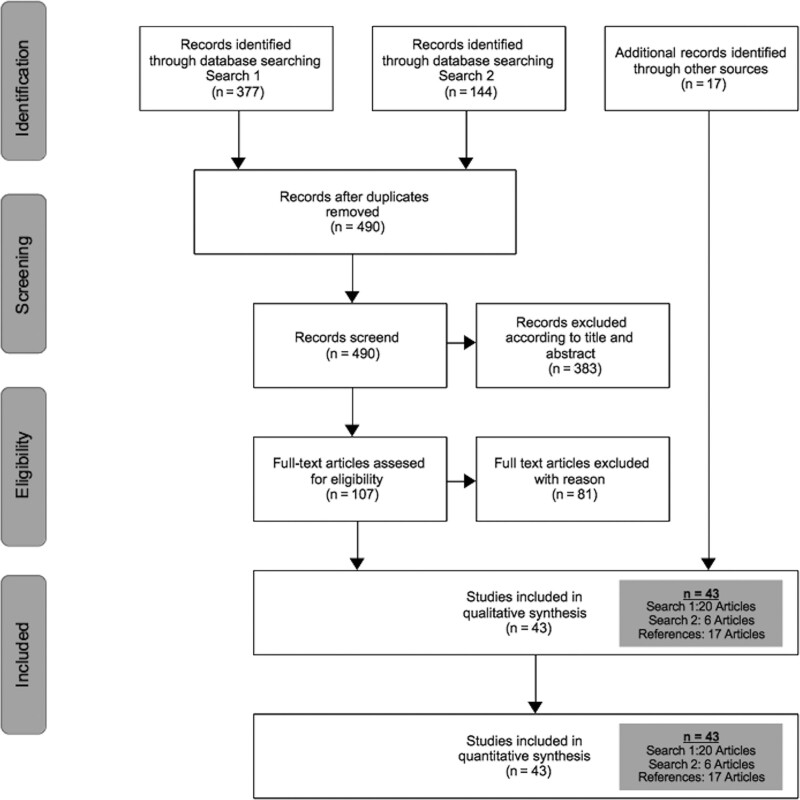
The systemic literature search strategy identified 377 publications in search 1 and 144 publications in search 2 (total 521). After removing duplicates (n = 31) and excluding clearly irrelevant records (n = 383), 107 full-text articles were retrieved for further investigation. Of these, 81 were excluded because they did not match the inclusion criteria. After exclusion of these articles and identification of 17 additional records through references, 43 studies were included in this meta-analysis. Article selection is summarized in Figure 1. Table 1 presents the baseline characteristics of the included publications involving 18,776 flaps in 17,532 patients. Altogether, 6332 flaps were performed in irradiated fields and 12,365 flaps in nonirradiated fields.
Fig. 1.
Diagram of the study selection process: from literature review to finally included articles.
Table 1.
Baseline Characteristics of Included Articles in Meta-analysis
| Author/Year/Study Type | Radiated Group | Nonradiated Group | No. Flaps | Reconstruction | Dose | Impact | Impact Timing | Comment on Significance of Flap Failure of Prior Radiotherapy | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (No. Patients) | Area | XRT Dose | Post Radiation | |||||||||
| Total | Partial | Total | Total | Partial | Total | On | On | |||||
| (Fracol et al. 2016) retro | Flap loss | Flap Loss | RX | Flap loss | Flap loss | No-RX | Flap Failure | Flap Failure | ||||
| (Fosnot et al. 2011) retro | 2 | n.r * | 199 | 4 | n.r | 199 | 398 (398) | Breast | n.r | n.r | n.r | Significant for intraoperative vascular complication (P 0.03) |
| (Chang et al. 2016) retro | 7 | n.r | 226 | 12 | n.r | 799 | 1025 (1025) | Breast | n.r | n.r | n.r | Significant for intraoperative vascular complication (P 0.003) |
| (Benatar et al. 2013) retro | 19 | 10 | 797 | 25 | 12 | 1336 | 2138 (1608) | Breast | n.r | n.r | n.r | n.s |
| (Momeni et al. 2011) retro | 19 | n.r | 136 | 26 | n.r | 293 | 429 (429) | H&N | ≤60 Gy; ≥60 Gy | + | n.r | ≥ 60 Gy significant for free flap failure (P 0.04) |
| (Zhou et al. 2017) retro | 0 | 7 | 34 | 0 | 1 | 26 | 60 (60) | H&N | n.r | n.r | n.r | Significant for flap-related complications (P 0.03)/higher rate of partial flap loss |
| (Ishimaru et al. 2016) retro | 7 | n.r | 55 | 19 | n.r | 826 | 881 (881) | H&N | ≥60 Gy | n.r | n.r | Significant for free flap failure (P 0.002) |
| (Tan et al. 2014) retro | 17 | n.r | 345 | 77 | n.r | 2501 | 2846 (2846) | H&N | n.r | n.r | n.r | Significant for free flap failure (P 0.022) |
| (Fracol et al. 2016) retro | 14 | n.r | 178 | 31 | n.r | 675 | 853 (782) | H&N | 50–63 Gy | n.r | n.r | n.s |
| (Sokoya et al. 2018) retro | 2 | n.r | 62 | 0 | n.r | 28 | 90 (45) | H&N | n.r | n.r | n.r | n.s |
| (Yu et al. 2009) retro | 13 | n.r | 577 | 14 | n.r | 733 | 1310 (1266) | H&N | n.r | n.r | n.r | n.s |
| (Arce et al. 2012) retro | 2 | 1 | 21 | 2 | 0 | 25 | 46 (46) | H&N | n.r | n.r | n.r | n.s |
| (Bengtson et al. 1993) pros | 9 | n.r | 169 | 10 | n.r | 199 | 368 (354) | H&N | 58–59 Gy | n.r | n.r | n.s |
| (S. Choi et al. 2004) retro | 0 | 3 | 37 | 0 | 2 | 28 | 65 (65) | H&N | 36–77 Gy | n.r | n.r | n.s |
| (Mücke et al. 2016) retro | 7 | n.r | 51 | 11 | n.r | 400 | 451 (451) | H&N | n.r | n.r | n.r | n.s |
| (Mulholland et al. 1993) retro | 8 | n.r | 226 | 3 | n.r | 108 | 334 (334) | H&N | 50–60 Gy | n.r | + | n.s |
| (Kiener et al. 1991) retro | 1 | n.r | 21 | 3 | n.r | 26 | 47 (42) | H&N | 60.9 Gy | n.r | n.r | n.s |
| (Aitasalo et al. 1997) retro | 10 | n.r | 77 | 1 | n.r | 11 | 88 (88) | H&N | 50–65 Gy | n.r | n.r | n.s |
| (Lin et al. 2005) retro | 1 | n.r | 44 | 0 | n.r | 70 | 114 (114) | H&N | 10–73.5 Gy | n.r | n.r | n.s |
| (Bozikov & Arnez 2006) retro | 8 | n.r | 45 | 11 | n.r | 89 | 194 (162) | H&N | n.r | n.r | n.r | n.s |
| (Halle et al. 2009) retro | 22 | 8 | 194 | 0 | 0 | 27 | 221 (216) | H&N | ≤54 Gy; 64 Gy | n.r | + | Significant for partial/total free flap failure (P 0.0296) |
| (Jones et al. 1996) retro | 13 | n.r | 113 | 14 | n.r | 192 | 305 (286) | H&N | n.r | n.r | n.r | n.s |
| (Klug et al. 2006) retro | 24 | n.r | 345 | 5 | n.r | 110 | 476 (455) | H&N | 50–≥ 60 Gy | n.r | n.r | n.s |
| (Tabah et al. 1984) retro | 4 | n.r | 41 | 1 | n.r | 34 | 75 (70) | H&N | n.r | n.r | n.r | n.s |
| (Nuara et al. 2009) pros | 1 | 3 | 160 | 0 | 1 | 140 | 300 (300) | H&N | n.r | n.r | n.r | n.s |
| (Ross et al. 2004) retro | 4 | n.r | 60 | 2 | n.r | 63 | 123 (120) | H&N | n.r | n.r | n.r | n.s |
| (Townley et al. 2013) retro | 1 | 1 | 32 | 0 | 1 | 14 | 46 (46) | Sarcoma defects | 50 Gy | n.r | n.r | n.s |
| (Azzi et al. 2019) retro | 1 | 1 | 8 | 1 | 2 | 8 | 16 (8) | Sarcoma defects | n.r | n.r | n.r | n.s |
| (Momoh et al. 2012) retro | 1 | 2 | 100 | 0 | 1 | 99 | 199 (199) | Breast | n.r | n.r | n.s | n.s |
| (Tran et al. 2001) retro | 1 | 5 | 70 | 0 | 0 | 32 | 102 (102) | Breast | 50–51 Gy | n.r | n.r | n.s |
| (Nahabedian & Momen 2008) retro | 2 | n.r | 60 | 0 | n.r | 39 | 99 (99) | Breast | 40–50 Gy | n.r | n.r | n.s |
| (J. W. Choi et al. 2020) retro | 7 | n.r | 95 | 1 | n.r | 557 | 652 (588) | H&N | n.r | n.r | n.r | Significant for free flap failure (P 0.001) |
| (Las et al. 2016) retro | n.r | 14 | 156 | n.r | 18 | 475 | 631 (458) | Breast | n.r | n.r | n.r | Significant for partial free flap failure (P 0.006) |
| (Mull et al. 2017) retro | 5 | 7 | 142 | 15 | 5 | 312 | 454 (331) | Breast | n.r | n.r | n.s | n.s |
| (Chao et al. 2012) retro | 0 | 2 | 73 | 4 | 0 | 46 | 119 (119) | Sarcoma defects | 50.3 ± 3 Gy | n.r | n.r | n.s |
| (Tall et al. 2015) retro | 29 | n.r | 283 | 1 | n.r | 61 | 344 (344) | H&N | 64 Gy | n.r | + | Significant for free flap failure (P 0.035) |
| (Maffi & Tran 2001) retro | 3 | n.r | 5 | 5 | n.r | 48 | 53 (49) | Not specified | n.r | n.r | n.r | Significant for free flap failure |
| (O’Neill et al. 2020) retro | 6 | n.r | 554 | 6 | n.r | 453 | 1012 (1012) | Breast | n.r | n.r | n.r | n.s |
| (Crawley et al. 2019) retro | 10 | n.r | 144 | 25 | n.r | 588 | 702 (702) | H&N | n.r | n.r | n.r | n.s |
| (Verhelst et al. 2019) retro | 9 | n.r | 48 | 7 | n.r | 24 | 72 (72) | H&N | n.r | n.r | n.r | n.s |
| (Khouri et al. 1998) pros | 5 | n.r | 53 | 15 | n.r | 440 | 493 (468) | Not specified | n.r | n.r | n.r | Significant for free flap failure (P 0.011) |
| (Schultze-Mosgau et al. 2002) retro | 15 | 11 | 123 | 3 | 3 | 76 | 217 (199) | H&N | 40–50 Gy; 60–70 Gy | + | n.s | Not available |
| (Hirsch et al. 2008) retro | 5 | 2 | 42 | 3 | 1 | 25 | 67 (67) | H&N | 66.5 Gy | n.r | n.r | n.s |
| (Hanasono et al. 2009) retro | 2 | 3 | 131 | 0 | 1 | 130 | 261 (226) | H&N | n.r | n.r | n.r | n.s |
+, significant; n.r, not recorded; n.s, not significant.
The patients were categorized in two groups, namely one group that had undergone preoperative XRT and microvascular free flap transfer and another group that had not experienced XRT before flap transfer.
Data Synthesis
The meta-analysis was conducted using MedCalc Statistical Software (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2020). Fixed-effect and random-effect models were applied to obtain the odds ratio for flap failure and partial flap failure between the radiation and the nonradiation groups. Ninety-five percent confidence intervals (CIs) of ODDS were also reported. Statistical heterogeneity was evaluated using Cochran’s Q test and Higgins I2 statistic. Forest plots were constructed graphically to illustrate the differences in outcome between the XRT and the non-XRT groups. The possibility of a particular publication bias affecting the results of the analysis was assessed visually using a funnel plot for asymmetry and by conducting Egger and Begg tests.
Meta-analysis
Our meta-analysis demonstrated statistically significantly increased risks for total flap failure and partial flap failure in the preirradiated group in fixed- and random-effect models.
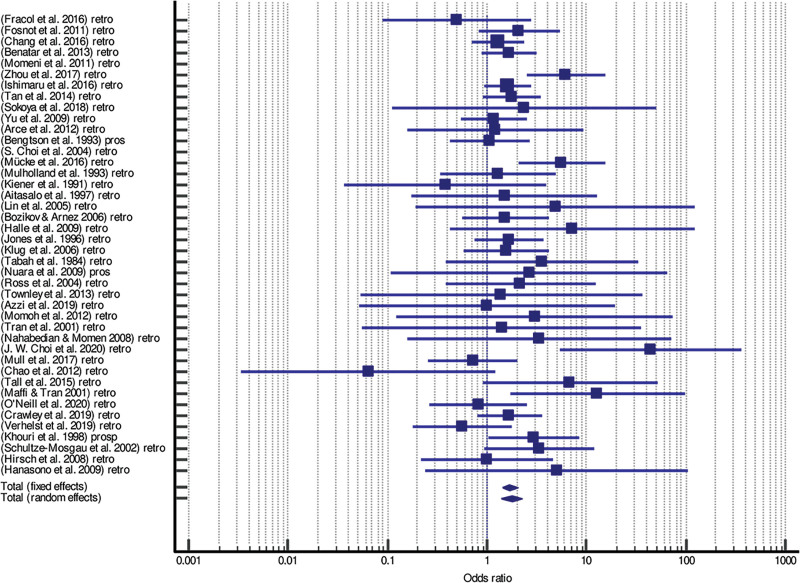
Patients with preoperative XRT were significantly associated with an increased risk for total (odds ratio fixed = 1.675, 95% CI = 1.405–1.996, P < 0.001; odds ratio random = 1.750, 95% CI = 1.386–2.208, P < 0.001) and partial free flap failure (odds ratio fixed = 2.161, 95% CI = 1.472–2.172, P < 0.001; odds ratio random = 2.112, 95% CI = 1.429–3.120, P < 0.001) (Tables 2 and 3).
Table 2.
Meta-analysis Results of Total Flap Failure
| Author/Year/Study Type | XRT Events/Total | Non-XRT Events/Total | Odds Ratio | 95% CI | Z | P | Weight (%) | |
|---|---|---|---|---|---|---|---|---|
| Fixed | Random | |||||||
| (Fracol et al. 2016) retro | 2/199 | 4/199 | 0.495 | 0.0896 to 2.733 | 1.10 | 1.59 | ||
| (Fosnot et al. 2011) retro | 7/226 | 12/799 | 2.096 | 0.815 to 5.389 | 3.62 | 3.93 | ||
| (Chang et al. 2016) retro | 19/797 | 25/1336 | 1.281 | 0.701 to 2.341 | 8.87 | 6.37 | ||
| (Benatar et al. 2013) retro | 19/136 | 26/293 | 1.668 | 0.888 to 3.132 | 8.12 | 6.13 | ||
| (Momeni et al. 2011) retro | 0/34 | 0/26 | — | |||||
| (Zhou et al. 2017) retro | 7/55 | 19/826 | 6.194 | 2.483 to 15.453 | 3.86 | 4.10 | ||
| (Ishimaru et al. 2016) retro | 17/345 | 77/2501 | 1.632 | 0.953 to 2.794 | 11.16 | 6.98 | ||
| (Tan et al. 2014) retro | 14/178 | 31/675 | 1.773 | 0.922 to 3.411 | 7.54 | 5.93 | ||
| (Sokoya et al. 2018) retro | 2/62 | 0/28 | 2.355 | 0.109 to 50.683 | 0.34 | 0.55 | ||
| (Yu et al. 2009) retro | 13/577 | 14/733 | 1.184 | 0.552 to 2.539 | 5.54 | 5.07 | ||
| (Arce et al. 2012) retro | 2/21 | 2/25 | 1.211 | 0.156 to 9.422 | 0.77 | 1.15 | ||
| (Bengtson et al. 1993) pros | 9/169 | 10/199 | 1.063 | 0.422 to 2.681 | 3.77 | 4.04 | ||
| (S. Choi et al. 2004) retro | 0/37 | 0/28 | — | |||||
| (Mücke et al. 2016) retro | 7/51 | 11/400 | 5.626 | 2.075 to 15.257 | 3.24 | 3.66 | ||
| (Mulholland et al. 1993) retro | 8/226 | 3/108 | 1.284 | 0.334 to 4.941 | 1.78 | 2.35 | ||
| (Kiener et al. 1991) retro | 1/21 | 3/26 | 0.383 | 0.0369 to 3.984 | 0.59 | 0.91 | ||
| (Aitasalo et al. 1997) retro | 10/77 | 1/11 | 1.493 | 0.172 to 12.947 | 0.69 | 1.05 | ||
| (Lin et al. 2005) retro | 1/44 | 0/70 | 4.862 | 0.194 to 122.042 | 0.31 | 0.50 | ||
| (Bozikov & Arnez 2006) retro | 8/45 | 11/89 | 1.533 | 0.569 to 4.131 | 3.28 | 3.69 | ||
| (Halle et al. 2009) retro | 22/194 | 0/27 | 7.174 | 0.423 to 121.716 | 0.40 | 0.64 | ||
| (Jones et al. 1996) retro | 13/113 | 14/192 | 1.653 | 0.747 to 3.655 | 5.12 | 4.85 | ||
| (Klug et al. 2006) retro | 24/345 | 5/110 | 1.570 | 0.584 to 4.219 | 3.30 | 3.70 | ||
| (Tabah et al. 1984) retro | 4/41 | 1/34 | 3.568 | 0.379 to 33.546 | 0.64 | 0.98 | ||
| (Nuara et al. 2009) pros | 1/160 | 0/140 | 2.643 | 0.107 to 65.396 | 0.31 | 0.50 | ||
| (Ross et al. 2004) retro | 4/60 | 2/63 | 2.179 | 0.384 to 12.359 | 1.07 | 1.55 | ||
| (Townley et al. 2013) retro | 1/32 | 0/14 | 1.381 | 0.0530 to 35.993 | 0.30 | 0.49 | ||
| (Azzi et al. 2019) retro | 1/8 | 1/8 | 1.000 | 0.0517 to 19.361 | 0.37 | 0.58 | ||
| (Momoh et al. 2012) retro | 1/100 | 0/99 | 3.000 | 0.121 to 74.539 | 0.31 | 0.50 | ||
| (Tran et al. 2001) retro | 1/70 | 0/32 | 1.403 | 0.0556 to 35.381 | 0.31 | 0.50 | ||
| (Nahabedian & Momen 2008) retro | 2/60 | 0/39 | 3.376 | 0.158 to 72.229 | 0.34 | 0.55 | ||
| (J. W. Choi et al. 2020) retro | 7/95 | 1/557 | 44.227 | 5.376 to 363.835 | 0.73 | 1.10 | ||
| (Mull et al. 2017) retro | 5/142 | 15/312 | 0.723 | 0.257 to 2.029 | 3.03 | 3.49 | ||
| (Chao et al. 2012) retro | 0/73 | 4/46 | 0.0642 | 0.00338 to 1.223 | 0.37 | 0.59 | ||
| (Tall et al. 2015) retro | 29/283 | 1/61 | 6.850 | 0.915 to 51.294 | 0.80 | 1.19 | ||
| (Maffi & Tran 2001) retro | 3/5 | 5/48 | 12.900 | 1.720 to 96.730 | 0.79 | 1.19 | ||
| (O’Neill et al. 2020) retro | 6/554 | 6/453 | 0.816 | 0.261 to 2.547 | 2.49 | 3.04 | ||
| (Crawley et al. 2019) retro | 10/144 | 25/588 | 1.681 | 0.788 to 3.583 | 5.63 | 5.11 | ||
| (Verhelst et al. 2019) retro | 9/48 | 7/24 | 0.560 | 0.179 to 1.753 | 2.48 | 3.03 | ||
| (Khouri et al. 1998) pros | 5/53 | 15/440 | 2.951 | 1.027 to 8.478 | 2.90 | 3.39 | ||
| (Schultze-Mosgau et al. 2002) retro | 15/123 | 3/76 | 3.380 | 0.945 to 12.091 | 1.99 | 2.57 | ||
| (Hirsch et al. 2008) retro | 5/42 | 3/25 | 0.991 | 0.216 to 4.556 | 1.39 | 1.92 | ||
| (Hanasono et al. 2009) retro | 2/131 | 0/130 | 5.039 | 0.240 to 105.982 | 0.35 | 0.55 | ||
| Total (fixed effects) | 316/6176 | 357/11.890 | 1.675 | 1.405 to 1.996 | 5.756 | <0.001 | 100.00 | 100.00 |
| Total (random effects) | 316/6176 | 357/11.890 | 1.750 | 1.386 to 2.208 | 4.712 | <0.001 | 100.00 | 100.00 |
Heterogeneity: Q = 53.2592, df = 39 (P = 0.0636); I2 = 26.77%/Publication bias: Egger test P = 0.4005; Begg test P = 0.4559.
Table 3.
Meta-analysis Results of Partial Flap Failure
| Author/Year/Study Type | XRT Events/ Total | Non-XRT Events/ Total | Odds Ratio | 95% CI | Z | P | Weight (%) | |
|---|---|---|---|---|---|---|---|---|
| Fixed | Random | |||||||
| (Chang et al. 2016) retro | 10/797 | 12/1336 | 1.402 | 0.603 to 3.260 | 21.39 | 21.39 | ||
| (Momeni et al. 2011) retro | 7/34 | 1/26 | 6.481 | 0.744 to 56.472 | 3.25 | 3.25 | ||
| (Arce et al. 2012) retro | 1/21 | 0/25 | 3.732 | 0.144 to 96.536 | 1.44 | 1.44 | ||
| (S. Choi et al. 2004) retro | 3/37 | 2/28 | 1.147 | 0.178 to 7.373 | 4.40 | 4.40 | ||
| (Halle et al. 2009) retro | 8/194 | 0/27 | 2.507 | 0.141 to 44.663 | 1.84 | 1.84 | ||
| (Nuara et al. 2009) pros | 3/160 | 1/140 | 2.656 | 0.273 to 25.830 | 2.94 | 2.94 | ||
| (Townley et al. 2013) retro | 1/32 | 1/14 | 0.419 | 0.0243 to 7.224 | 1.88 | 1.88 | ||
| (Azzi et al. 2019) retro | 1/8 | 2/8 | 0.429 | 0.0307 to 5.985 | 2.19 | 2.19 | ||
| (Momoh et al. 2012) retro | 2/100 | 1/99 | 2.000 | 0.178 to 22.419 | 2.61 | 2.61 | ||
| (Tran et al. 2001) retro | 5/70 | 0/32 | 5.458 | 0.293 to 101.756 | 1.78 | 1.78 | ||
| (Las et al. 2016) retro | 14/156 | 18/475 | 2.503 | 1.214 to 5.160 | 29.10 | 29.10 | ||
| (Mull et al. 2017) retro | 7/142 | 5/312 | 3.184 | 0.993 to 10.210 | 11.21 | 11.21 | ||
| (Chao et al. 2012) retro | 2/73 | 0/46 | 3.252 | 0.153 to 69.268 | 1.63 | 1.63 | ||
| (Schultze-Mosgau et al. 2002) retro | 11/123 | 3/76 | 2.390 | 0.645 to 8.859 | 8.87 | 8.87 | ||
| (Hirsch et al. 2008) retro | 2/42 | 1/25 | 1.200 | 0.103 to 13.951 | 2.53 | 2.53 | ||
| (Hanasono et al. 2009) retro | 3/131 | 1/130 | 3.023 | 0.310 to 29.452 | 2.94 | 2.94 | ||
| Total (fixed effects) | 80/2120 | 48/2799 | 2.161 | 1.472 to 3.172 | 3.934 | <0.001 | 100.00 | 100.00 |
| Total (random effects) | 80/2120 | 48/2799 | 2.112 | 1.429 to 3.120 | 3.754 | <0.001 | 100.00 | 100.00 |
Heterogeneity: Q = 6.6836, df = 15 (P = 0.9658); I2 = 0%/Publication bias: Egger test P = 0.9084; Begg test P = 0.8571.
According to total flap failures in the preirradiated group, the test value for heterogeneity presented a Q value of 53.2592 with 39 df, P = 0.0636 and I2 26.77%.
Although I2 indicated only little heterogeneity but a significant P value, the random-effect model would be more appropriate regarding the significance of the effect of XRT on free flap failure, which also showed a significantly increased risk for total free flap failures in preirradiated patients (odds ratio random = 1.750, 95% CI = 1.386–2.208, P < 0.001).
Outcomes of partial free flap failure after radiotherapy show an absence of heterogeneity among the studies (Q value 6.6836 with 15 df, P = 0.9658, I2 0%).
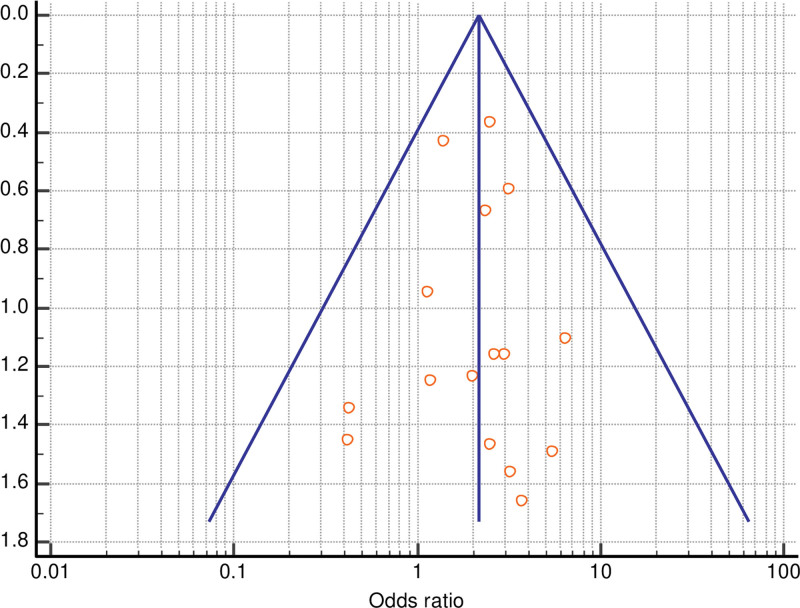
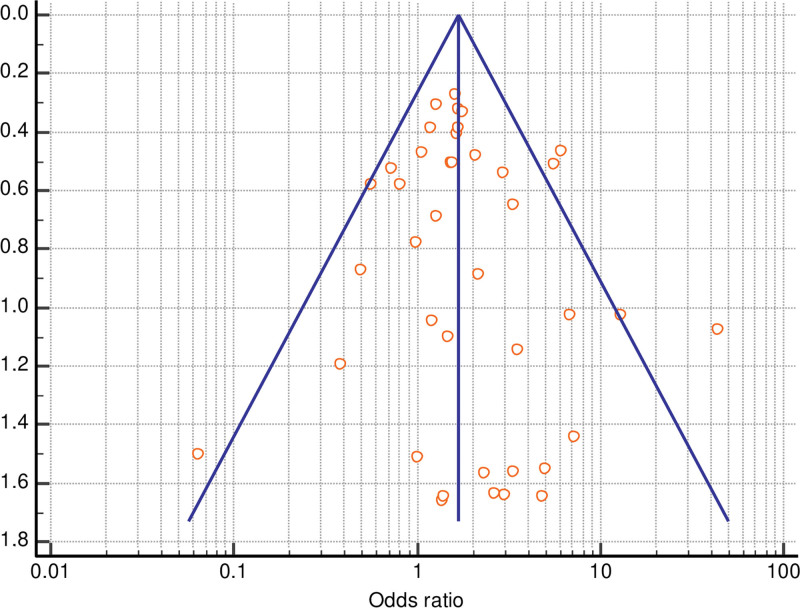
The Egger test and Begg test were not significant for publication bias for total (Egger test P = 0.4005; Begg test P = 0.4559) or partial free flap failure (Egger test P = 0.9084; Begg test P = 0.8571), and funnel plots showed basic symmetry.
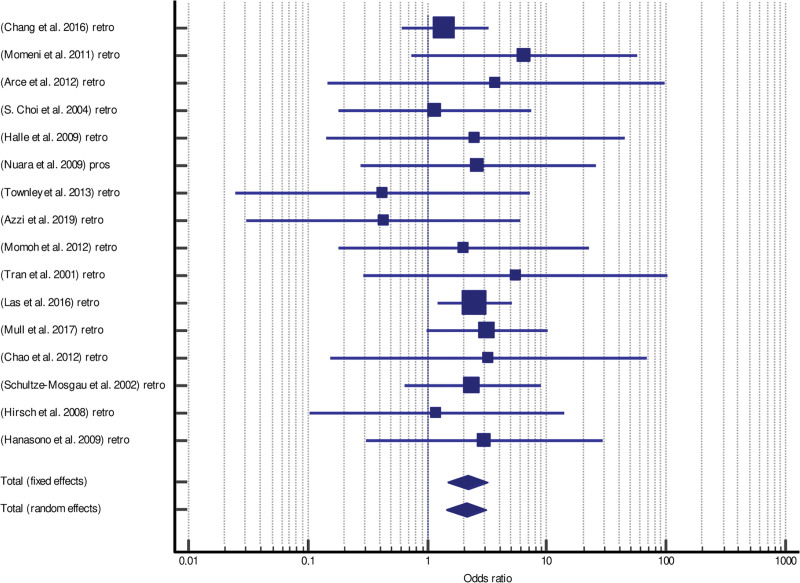
Forrest plots and funnel plots for total and partial flap failure are shown in Figures 2–5.
Fig. 2.
Forrest plot of meta-analysis of total flap failure.
Fig. 5.
Funnel plot of meta-analysis of partial flap failure.
Fig. 3.
Funnel plot of meta-analysis of total flap failure.
Fig. 4.
Forrest plot of meta-analysis of partial flap failure.
Additionally, we also compared the risk for total free flap failure between breast and head and neck patients. Preirradiated head and neck patients showed a significantly increased risk for total flap failure (odds ratio fixed = 1.858, 95% CI = 1.515–2.278, P < 0.001; odds ratio random = 1.902, 95% CI = 1.460–2.479, P < 0.001) compared to breast patients (odds ratio fixed = 1.172, 95% CI = 0.789–1.740, P = 0.433; odds ratio random = 1.189, 95% CI = 0.796–1.775, P = 0.398).
Outcomes for total free flap failure after radiotherapy in those groups show an absence of heterogeneity among the studies with no significant publication bias in breast patients (Q value 4.549 with 7 df, P = 0.7148, I2 0%/Egger test P = 0.9050; Begg test P = 0.8046) and head and neck patients (Q value 35.252 with 26 df, P = 0.106, I2 26.25%/Egger test P = 0.1937; Begg test P = 0.1625).
DISCUSSION
The impact of previous radiotherapy on free flap outcome is the subject of ongoing debate, because there have been conflicting reports on the incidence of free flap failure following radiotherapy. Most clinical evidence related to free flap reconstruction in irradiated fields derives from head and neck reconstruction. Because of the observational character of the studies they are prone to a variety of biases, especially confounding. Different comorbidities and individual risk factors might have an effect on the outcome of free flap reconstruction. Thus, the inclusion of those factors might impact the rate of free flap failures.
A Matter of Dissection
To delineate the direct effects of radiation one interesting study designed by Fracol et al. searched for patients who underwent bilateral breast free flap reconstruction following unilateral breast radiation. With this study design the nonradiated chest wall can be used as a matched control and any intrinsic characteristics of the patient that could contribute to an increased risk can be subtracted. Radiated fields were statistically significantly more likely than nonradiated fields to have intraoperative vascular complications related to arterial or venous thrombosis and abnormalities in the recipients, thus necessitating reperformance of arterial anastomosis.24 Fosnot et al. also reported that there appears to be a trend to higher anastomotic revision rates and the need for additional dissection of the recipient vessel to reach a usable target. He postulated that there may be an increase in vascular complications from progressive sclerosis in the long term, because trends show progressive coronary artery disease long after toxic effects of radiotherapy or environmental exposure.25,57 From a histological perspective lesions in medium-sized to large vessels (>100 µm in diameter) exhibit typical features of atherosclerosis and are indistinguishable from those that occur as a result of the generalized spontaneous process of atherosclerosis.57 Long-term changes are similar to an atherosclerotic process with intimal thickening and lipid accumulation.8 Irradiated vessels show increased wall thickness, intimal dehiscence, diminished smooth muscle density, accumulation of lipid-laden macrophages in the intimal, vessel wall fibrosis and a large amount of periadventitial fibrosis.3,58 Inflammatory and proliferative cytokines are overexpressed after radiation leading to uncontrolled matrix accumulation and fibrosis in radiated fields.59 The increased wall thickness makes the arteries seem stiff during microsurgical dissection and the fibrosis can create an unfavorable environment for reconstructive surgery.3 Severe atherosclerosis makes recipient vessels vulnerable to traumatic injury and excessive tension can create fractures in the plaque or induce damage to the vessel wall.60 Therefore, irradiated vessels are friable and predisposed to arterial wall dissection.3 Consequently, intraoperative complications may be the result of technical difficulty in working with radiated vessels. The deleterious effect of previous radiation on vascular anastomoses appears to be related to intraoperative technical variation requiring more tedious dissection or revision maneuvers.25 It has been shown that intraoperative vascular events such as anastomotic revisions are associated with increased rates of flap loss.61,62 Although Fracol et al and Fosnot et al noted a significant correlation between intraoperative complications and radiation, no ultimate difference in the rate of flap loss between radiated and nonradiated fields undergoing free flap reconstruction was observed. However, Fracol et al noted that the small difference may be due to the heightened awareness and diligence when operating in radiated fields. Consequently, the results may be influenced by performance bias.
A Matter of Dose
Thankappan et al mentioned that there are many other variables in the method of administering radiotherapy that can potentially affect the outcome of free flap surgery, but that are not analyzed in the medical literature. These variables include the dose of radiation, type of radiation (conventional, protons, neutrons, and brachytherapy); the impact of intensity-modulated radiation therapy (IMRT) and altered fractionation schemes. These variables have a well-documented impact on the acute and long-term effects of treatment that might also impact the outcome of the reconstructive microvascular surgery.63 In particular, the reported conflicting results concerning the risk for free flap failure following radiotherapy may be influenced by differences in applied radiation doses. Especially, the dose of radiation may be a key factor in the outcome of free flap surgery after radiation. Changes in vasculature induced by radiation were shown to be dose-dependent. Significant histological changes in the recipient vessel walls were observed for radiation doses greater than 60 Gy, although no significant changes were observed for doses between 40 and 50 Gy. After a dose of 60–70 Gy significant intima detachments and media thickening are detectable in the arteries, while radiation with a dose between 40 and 50 Gy did not result in changes in the vessel walls.23 Patients with these vascular changes in the connecting vessels showed an increased rate of total and partial flap loss. The flap success percentage decreased from 94% in nonirradiated patients and over 90% in patients who had received a radiation dose less than 50 Gy to 84% in patients who had received a radiation dose between 60 and 70 Gy.23 A significant correlation between total doses exceeding 60 Gy in head and neck reconstruction and flap failure was also specifically noted by Benatar et al. This is in agreement with the results of our meta-analysis and the observation by Kroll et al of an association between preoperative radiotherapy and free flap failure in head and neck reconstruction, but shows no significant relationship in breast reconstructions, where lower radiation doses are commonly applied.64
A Matter of Timing
Further reasons for the reported discrepancies in the literature may be different time intervals between radiotherapy and surgery. The time interval that elapses between radiotherapy and reconstruction may additionally play a role. Only a few studies have examined the impact that timing following radiation has on the outcome of free flap reconstruction.
In head and neck reconstruction, Mulholland et al examined timing after neoadjuvant radiotherapy and demonstrated a linear relationship between time elapsed after radiation and the incidence of free flap loss.34
More precisely, Halle et al also found an increased rate of flap necrosis in the same population of patients when surgery was performed more than 6 weeks postradiotherapy.17
In accordance therewith, Tall et al reported an increase in free flap success in patients who underwent head and neck reconstruction within 6 weeks after radiotherapy compared to delayed (6–12 weeks) and late reconstructions (>15 weeks). The total flap failure rate steadily increased with the time elapsed since the last radiotherapy session.20
Nevertheless, the analysis of breast patients reconstructed before and after 6 months and more and less than 12 months after radiation revealed no significant differences in flap loss.46,49
In contrast, one study reported a lower rate of flap failure in patients who underwent delayed free autologous breast reconstruction more than 12 months after completion of postmastectomy radiation therapy as compared with the group who underwent this surgery within 12 months.65
However, except for the study by Baumann et al the observed results of a significant association between time interval and free flap failure in head and neck reconstruction as compared to failed association in breast reconstruction, where patients are usually exposed to lower radiation doses, substantiate the assumption that the impact of previous radiotherapy on free flap outcome is a function of total dose and time after radiation.
Adverse acute effects of radiation usually occur within 4–6 weeks after irradiation,2,7 and studies suggest that a shorter time interval to reconstruction may increase flap survival rates. The beneficial role of early reconstruction after radiotherapy may be explained by the initial latent period before vascular damage occurs together with the delayed adverse effects of blood vessels after irradiation.20
CONCLUSIONS
The results of this meta-analysis suggest that preoperative radiotherapy is significantly associated with an increased risk for total and partial free flap failure. Further outcome studies are needed, especially to investigate the effect of total XRT dose and time after radiation on free flap outcome.
Addendum
The study was done as the Master Thesis of the International Master’s Degree in Reconstructive Microsurgery of the Reconstructive Microsurgery European School.
ACKNOWLEDGMENT
We thank Mary Heaney Margreiter for critical reading and editorial assistance.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Hubenak JR, Zhang Q, Branch CD, et al. Mechanisms of injury to normal tissue after radiotherapy: a review. Plast Reconstr Surg. 2014;133:49e–56e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone HB, Coleman CN, Anscher MS, et al. Effects of radiation on normal tissue: consequences and mechanisms. Lancet Oncol. 2003;4:529–536. [DOI] [PubMed] [Google Scholar]
- 3.Guelinckx PJ, Boeckx WD, Fossion E, et al. Scanning electron microscopy of irradiated recipient blood vessels in head and neck free flaps. Plast Reconstr Surg. 1984;74:217–226. [DOI] [PubMed] [Google Scholar]
- 4.Gaugler M-H. A unifying system: does the vascular endothelium have a role to play in multi-organ failure following radiation exposure? Br J of Radiol Suppl. 2005;27:100–105. [Google Scholar]
- 5.Vereycken-Holler V, Aigueperse J, Gaugler MH. Radiation effects on circulating and endothelial cell interactions studied by quantitative real-time videomicroscopy. Int J Radiat Biol. 2002;78:923–930. [DOI] [PubMed] [Google Scholar]
- 6.Halle M, Ekström M, Farnebo F, et al. Endothelial activation with prothrombotic response in irradiated microvascular recipient veins. J Plast Reconstr Aesthet Surg. 2010;63:1910–1916. [DOI] [PubMed] [Google Scholar]
- 7.Halle M, Gabrielsen A, Paulsson-Berne G, et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol. 2010;55:1227–1236. [DOI] [PubMed] [Google Scholar]
- 8.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. [DOI] [PubMed] [Google Scholar]
- 9.Cohen EP, Fish BL, Moulder JE. The role of nitric oxide in radiation nephropathy. Arch Physiol Biochem. 1996;104:200–206. [DOI] [PubMed] [Google Scholar]
- 10.Azizzadeh B, Buga GM, Berke GS, et al. Inhibitors of nitric oxide promote microvascular thrombosis. Arch Facial Plast Surg. 2003;5:31–35. [DOI] [PubMed] [Google Scholar]
- 11.Krag C, De Rose G, Lyczakowski T, et al. Free flaps and irradiated recipient vessels: an experimental study in rabbits. Br J Plast Surg. 1982;35:328–336. [DOI] [PubMed] [Google Scholar]
- 12.Krag C, Holck S, DeRose G, et al. Healing of microvascular anastomoses. A comparative study using normal and irradiated recipient vessels for experimental free flaps in rabbits. Scand J Plast Reconstr Surg. 1982;16:267–274. [DOI] [PubMed] [Google Scholar]
- 13.Benatar MJ, Dassonville O, Chamorey E, et al. Impact of preoperative radiotherapy on head and neck free flap reconstruction: a report on 429 cases. J Plast Reconstr Aesthet Surg. 2013;66:478–482. [DOI] [PubMed] [Google Scholar]
- 14.Momeni A, Kim RY, Kattan A, et al. The effect of preoperative radiotherapy on complication rate after microsurgical head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2011;64:1454–1459. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Zhang WB, Yu Y, et al. Risk factors for free flap failure: a retrospective analysis of 881 free flaps for head and neck defect reconstruction. Int J Oral Maxillofac Surg. 2017;46:941–945. [DOI] [PubMed] [Google Scholar]
- 16.Ishimaru M, Ono S, Suzuki S, et al. Risk factors for free flap failure in 2,846 patients with head and neck cancer: a national database study in Japan. J Oral Maxillofac Surg. 2016;74:1265–1270. [DOI] [PubMed] [Google Scholar]
- 17.Halle M, Bodin I, Tornvall P, et al. Timing of radiotherapy in head and neck free flap reconstruction–a study of postoperative complications. J Plast Reconstr Aesthet Surg. 2009;62:889–895. [DOI] [PubMed] [Google Scholar]
- 18.Choi JW, Kim YC, Jeon DN, et al. Impact of recipient vein selection on venous patency and free flap survival in 652 head and neck reconstructions. J Reconstr Microsurg. 2020;36:73–81. [DOI] [PubMed] [Google Scholar]
- 19.Las DE, de Jong T, Zuidam JM, et al. Identification of independent risk factors for flap failure: a retrospective analysis of 1530 free flaps for breast, head and neck and extremity reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:894–906. [DOI] [PubMed] [Google Scholar]
- 20.Tall J, Björklund TC, Skogh AC, et al. Vascular complications after radiotherapy in head and neck free flap reconstruction: clinical outcome related to vascular biology. Ann Plast Surg. 2015;75:309–315. [DOI] [PubMed] [Google Scholar]
- 21.Maffi TR, Tran NV. Free-tissue transfer experience at a county hospital. J Reconstr Microsurg. 2001;17:431–433. [DOI] [PubMed] [Google Scholar]
- 22.Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102:711–721. [DOI] [PubMed] [Google Scholar]
- 23.Schultze-Mosgau S, Grabenbauer GG, Radespiel-Tröger M, et al. Vascularization in the transition area between free grafted soft tissues and pre-irradiated graft bed tissues following preoperative radiotherapy in the head and neck region. Head Neck. 2002;24:42–51. [DOI] [PubMed] [Google Scholar]
- 24.Fracol ME, Basta MN, Nelson JA, et al. Bilateral free flap breast reconstruction after unilateral radiation: comparing intraoperative vascular complications and postoperative outcomes in radiated versus nonradiated breasts. Ann Plast Surg. 2016;76:311–314. [DOI] [PubMed] [Google Scholar]
- 25.Fosnot J, Fischer JP, Smartt JM, Jr, et al. Does previous chest wall irradiation increase vascular complications in free autologous breast reconstruction? Plast Reconstr Surg. 2011;127:496–504. [DOI] [PubMed] [Google Scholar]
- 26.Chang EI, Chang EI, Soto-Miranda MA, et al. Comprehensive evaluation of risk factors and management of impending flap loss in 2138 breast free flaps. Ann Plast Surg. 2016;77:67–71. [DOI] [PubMed] [Google Scholar]
- 27.Tan NC, Lin PY, Chiang YC, et al. Influence of neck dissection and preoperative irradiation on microvascular head and neck reconstruction-Analysis of 853 cases. Microsurgery. 2014;34:602–607. [DOI] [PubMed] [Google Scholar]
- 28.Sokoya M, Bahrami A, Vincent A, et al. Preoperative radiation and complication rates after double free flap reconstruction of head and neck cancer. Am J Otolaryngol. 2018;39:558–560. [DOI] [PubMed] [Google Scholar]
- 29.Yu P, Chang DW, Miller MJ, et al. Analysis of 49 cases of flap compromise in 1310 free flaps for head and neck reconstruction. Head Neck. 2009;31:45–51. [DOI] [PubMed] [Google Scholar]
- 30.Arce K, Bell RB, Potter JK, et al. Vascularized free tissue transfer for reconstruction of ablative defects in oral and oropharyngeal cancer patients undergoing salvage surgery following concomitant chemoradiation. Int J Oral Maxillofac Surg. 2012;41:733–738. [DOI] [PubMed] [Google Scholar]
- 31.Bengtson BP, Schusterman MA, Baldwin BJ, et al. Influence of prior radiotherapy on the development of postoperative complications and success of free tissue transfers in head and neck cancer reconstruction. Am J Surg. 1993;166:326–330. [DOI] [PubMed] [Google Scholar]
- 32.Choi S, Schwartz DL, Farwell DG, et al. Radiation therapy does not impact local complication rates after free flap reconstruction for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:1308–1312. [DOI] [PubMed] [Google Scholar]
- 33.Mücke T, Ritschl LM, Roth M, et al. Predictors of free flap loss in the head and neck region: a four-year retrospective study with 451 microvascular transplants at a single centre. J Craniomaxillofac Surg. 2016;44:1292–1298. [DOI] [PubMed] [Google Scholar]
- 34.Mulholland S, Boyd JB, McCabe S, et al. Recipient vessels in head and neck microsurgery: radiation effect and vessel access. Plast Reconstr Surg. 1993;92:628–632. [DOI] [PubMed] [Google Scholar]
- 35.Kiener JL, Hoffman WY, Mathes SJ. Influence of radiotherapy on microvascular reconstruction in the head and neck region. Am J Surg. 1991;162:404–407. [DOI] [PubMed] [Google Scholar]
- 36.Aitasalo K, Relander M, Virolainen E. Microvascular free tissue transfers after preoperative irradiation in head and neck reconstructions. Acta Otolaryngol Suppl. 1997;529:247–250. [DOI] [PubMed] [Google Scholar]
- 37.Lin S, Dutra J, Keni J, et al. Preoperative radiation therapy and its effects on outcomes in microsurgical head and neck reconstruction. Otolaryngol Head Neck Surg. 2005;132:845–848. [DOI] [PubMed] [Google Scholar]
- 38.Bozikov K, Arnez ZM. Factors predicting free flap complications in head and neck reconstruction. J Plast Reconstr Aesthet Surg. 2006;59:737–742. [DOI] [PubMed] [Google Scholar]
- 39.Jones NF, Johnson JT, Shestak KC, et al. Microsurgical reconstruction of the head and neck: interdisciplinary collaboration between head and neck surgeons and plastic surgeons in 305 cases. Ann Plast Surg. 1996;36:37–43. [DOI] [PubMed] [Google Scholar]
- 40.Klug C, Berzaczy D, Reinbacher H, et al. Influence of previous radiotherapy on free tissue transfer in the head and neck region: evaluation of 455 cases. Laryngoscope. 2006;116:1162–1167. [DOI] [PubMed] [Google Scholar]
- 41.Tabah RJ, Flynn MB, Acland RD, et al. Microvascular free tissue transfer in head and neck and esophageal surgery. Am J Surg. 1984;148:498–504. [DOI] [PubMed] [Google Scholar]
- 42.Nuara MJ, Sauder CL, Alam DS. Prospective analysis of outcomes and complications of 300 consecutive microvascular reconstructions. Arch Facial Plast Surg. 2009;11:235–239. [DOI] [PubMed] [Google Scholar]
- 43.Ross DA, Hundal JS, Son YH, et al. Microsurgical free flap reconstruction outcomes in head and neck cancer patients after surgical extirpation and intraoperative brachytherapy. Laryngoscope. 2004;114:1170–1176. [DOI] [PubMed] [Google Scholar]
- 44.Townley WA, Mah E, O’Neill AC, et al. Reconstruction of sarcoma defects following pre-operative radiation: free tissue transfer is safe and reliable. J Plast Reconstr Aesthet Surg. 2013;66:1575–1579. [DOI] [PubMed] [Google Scholar]
- 45.Azzi AJ, Zhou S, Safran T, et al. Vascularized tissue reconstruction in previously irradiated sarcoma defects. Ann Plast Surg. 2019;82:89–92. [DOI] [PubMed] [Google Scholar]
- 46.Momoh AO, Colakoglu S, de Blacam C, et al. Delayed autologous breast reconstruction after postmastectomy radiation therapy: is there an optimal time? Ann Plast Surg. 2012;69:14–18. [DOI] [PubMed] [Google Scholar]
- 47.Tran NV, Chang DW, Gupta A, et al. Comparison of immediate and delayed free TRAM flap breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2001;108:78–82. [DOI] [PubMed] [Google Scholar]
- 48.Nahabedian MY, Momen B. The impact of breast reconstruction on the oncologic efficacy of radiation therapy: a retrospective analysis. Ann Plast Surg. 2008;60:244–250. [DOI] [PubMed] [Google Scholar]
- 49.Mull AB, Qureshi AA, Zubovic E, et al. Impact of time interval between radiation and free autologous breast reconstruction. J Reconstr Microsurg. 2017;33:130–136. [DOI] [PubMed] [Google Scholar]
- 50.Chao AH, Chang DW, Shuaib SW, et al. The effect of neoadjuvant versus adjuvant irradiation on microvascular free flap reconstruction in sarcoma patients. Plast Reconstr Surg. 2012;129:675–682. [DOI] [PubMed] [Google Scholar]
- 51.O’Neill AC, Yang D, Roy M, et al. Development and evaluation of a machine learning prediction model for flap failure in microvascular breast reconstruction. Ann Surg Oncol. 2020;27:3466–3475. [DOI] [PubMed] [Google Scholar]
- 52.Crawley MB, Sweeny L, Ravipati P, et al. Factors associated with free flap failures in head and neck reconstruction. Otolaryngol Head Neck Surg. 2019;161:598–604. [DOI] [PubMed] [Google Scholar]
- 53.Verhelst PJ, Dons F, Van Bever PJ, et al. Fibula free flap in head and neck reconstruction: identifying risk factors for flap failure and analysis of postoperative complications in a low volume setting. Craniomaxillofac Trauma Reconstr. 2019;12:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirsch DL, Bell RB, Dierks EJ, et al. Analysis of microvascular free flaps for reconstruction of advanced mandibular osteoradionecrosis: a retrospective cohort study. J Oral Maxillofac Surg. 2008;66:2545–2556. [DOI] [PubMed] [Google Scholar]
- 55.Hanasono MM, Barnea Y, Skoracki RJ. Microvascular surgery in the previously operated and irradiated neck. Microsurgery. 2009;29:1–7. [DOI] [PubMed] [Google Scholar]
- 56.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fajardo LF. Is the pathology of radiation injury different in small vs large blood vessels? Cardiovasc Radiat Med. 1999;1:108–110. [DOI] [PubMed] [Google Scholar]
- 58.Fajardo LF. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005;44:13–22. [DOI] [PubMed] [Google Scholar]
- 59.Haubner F, Ohmann E, Pohl F, et al. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cigna E, Lo Torto F, Parisi P, et al. Management of microanastomosis in patients affected by vessel diseases. Eur Rev Med Pharmacol Sci. 2014;18:3399–3405. [PubMed] [Google Scholar]
- 61.Fosnot J, Jandali S, Low DW, et al. Closer to an understanding of fate: the role of vascular complications in free flap breast reconstruction. Plast Reconstr Surg. 2011;128:835–843. [DOI] [PubMed] [Google Scholar]
- 62.Chao AH, Coriddi M. The impact of intraoperative microvascular compromise on outcomes in microsurgical breast reconstruction. J Reconstr Microsurg. 2015;31:493–499. [DOI] [PubMed] [Google Scholar]
- 63.Thankappan K. Microvascular free tissue transfer after prior radiotherapy in head and neck reconstruction - a review. Surg Oncol. 2010;19:227–234. [DOI] [PubMed] [Google Scholar]
- 64.Kroll SS, Robb GL, Reece GP, et al. Does prior irradiation increase the risk of total or partial free-flap loss? J Reconstr Microsurg. 1998;14:263–268. [DOI] [PubMed] [Google Scholar]
- 65.Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg. 2011;127:1100–1106. [DOI] [PubMed] [Google Scholar]