Abstract
INTRODUCTION
Abdominal Aortic Aneurysm (AAA) is a common progressive disease and a significant cause of morbidity and mortality. Prior investigations have shown that diabetes mellitus (DM) may be relatively protective of AAA incidence and growth. The Non-invasive Treatment of Aortic Aneurysm Clinical Trial (N-TA3CT) is a contemporary study of small AAA growth which provides a unique opportunity to validate and explore the effect of DM on AAA. Confirming the effect of DM on AAA growth in this study may present opportunities to explore for clues to potential biologic mechanisms as well as inform current patient management.
METHODS
This is a secondary analysis examining the association of diabetes and aneurysm growth within N-TA3CT: a placebo-controlled multicenter trial of doxycycline in 261 patients with AAA maximum transverse diameters (MTD) between 3.5cm and 5cm. The primary outcome is the change in the MTD from baseline as determined by CT scans obtained during the trial. Secondary outcome is the growth pattern of the AAA. Baseline characteristics and growth patterns were assessed with t-tests (continuous) or chi-square tests (categorical). Unadjusted and adjusted longitudinal analyses were performed with a repeated measures linear mixed model to compare AAA growth rates between diabetic patients and non-diabetic patients.
RESULTS
Of 261 patients, 250 subjects had sufficient imaging and were included in this study. There were 56 (22.4%) with diabetes and 194 (77.6%) without. Diabetes was associated with higher BMI and increased rates of hypercholesterolemia and coronary artery disease (p< 0.05). Diabetes was also associated with increased frequency of treatment for atherosclerosis and hypertension including treatment with statin, angiotensin-converting enzyme inhibitor, ARB, anti-platelet, and diuretic therapy (p< 0.05). Baseline MTD was not significantly different between those with (4.32 cm) and without diabetes (4.30 cm). Median growth rate for diabetic patients was 0.12 cm/year (IQR 0.07-0.22) and 0.19 cm/year (IQR 0.12-0.27) in patients without diabetes, which was significantly different on unadjusted analysis (p<0.0001). Diabetes remained significantly associated with AAA growth after adjustment for other relevant clinical factors (coef −0.057; p<0.0001).
CONCLUSIONS
Patients with diabetes have more than a 35% reduction in the median growth rates of AAA despite more severe concomitant vascular co-morbidities and similar initial sizes of aneurysms. This effect persists and remains robust after adjusted analysis; and slower growth rates may delay the time to reach repair threshold. Rapid growth (>0.5 cm/year) is infrequent in diabetic patients.
Keywords: Vascular Surgery, Abdominal Aortic Aneurysm, Diabetes Mellitus, Doxycycline, Inflammation
Table of Contents Summary
In this retrospective analysis of a prospective, multicenter double-blind placebo-controlled trial, diabetic patients with abdominal aortic aneurysms exhibited slower aneurysm growth compared to non-diabetic patients. The diagnosis of diabetes is associated with an overall reduction in aneurysm growth rate despite more severe concomitant vascular co-morbidities and similar initial sizes of aneurysms.
INTRODUCTION
Abdominal Aortic Aneurysm (AAA) is a common progressive disease affecting over 1,000,000 people in the United States with a prevalence ranging from two to eight percent.1,2 It is a significant cause of morbidity and mortality, particularly in the elderly population.3 Risk factors for AAA incidence largely coincide with those of atherosclerosis.1 Although diabetes mellitus (DM) is a leading risk factor in atherosclerotic vascular disease, it may not exacerbate the AAA. Some but not all prior epidemiologic studies have demonstrated a negative association with AAA incidence, diameter, and growth.4–8 There is no consensus on why AAA may be resistant to the pathologic vascular consequences of DM. Investigation of this apparent paradoxical relationship has the potential to better direct investigations into understanding the biology of AAA growth with the hope of identifying targets for intervention. Further, understanding how these diseases interact may be important to the design considerations of future studies of AAA growth, particularly interventional trials. We performed a retrospective analysis of the association of diabetes with aneurysm growth based on a recently published clinical trial of patients with AAA.
METHODS
Data Source
The Non-invasive Treatment of Aortic Aneurysm Clinical Trial (N-TA3CT, NCT01756833) is a double-blind placebo-controlled trial of 261 (22% with diabetes) patients ages 55 and older with AAAs at enrollment between 3.5cm and 5cm for men and 3.5 cm to 4.5 cm for women investigating the effects of doxycycline on aneurysm growth over a 24-month period.9,10 Designed with conservative assumptions of AAA measurement reliability to have 85 informative patients at trial conclusion, N-TA3CT was expected to have a 90% probability of detecting a 40% difference in growth between treated and untreated patients. Follow-up included abdominal CT imaging and serum and plasma collection scheduled every 6 months.9 In N-TA3CT, doxycycline had no effect on aneurysm growth.10 After enrollment qualification based on initial CT imaging review, baseline clinical and demographic data were collected as well as peripheral blood specimens. Patients were followed for at least 24 months or until completion of the study, aneurysm repair, or study termination. Imaging of the aorta with CT scan and phlebotomy were scheduled every 6 months during follow-up.
Study protocol was approved by IRBs at all clinical sites, core laboratories and coordinating centers. Informed consent was obtained from all participants.
Growth Rate Assessment
CT image acquisition protocol was standardized across sites with image slice thickness requirements of ≤ 2.5 mm. AAA maximum transverse diameter (MTD) was evaluated at the Imaging Core Laboratory (ICL) at the University of Wisconsin, Madison. A single reader used the double oblique technique to measure the MTD.9,11,12 Patients were included in our primary growth analysis if there were at least 2 CT scans of the abdomen available for analysis in the ICL. MTD growth rates were calculated based on estimation from a linear regression slope of all available CT scans and were expressed as centimeters of growth per year (cm/yr).
Growth Pattern Assessment
A detailed characterization of growth patterns has been described for the N-TA3CT participants.13 Subjects with a baseline CT scan and at least 3 additional CT scans through at least 18 months were included in the growth pattern analysis. Regression modelling of aneurysm diameter on time and growth pattern characterization was performed as defined by Olson et al.13 If subjects did not have linear growth, they were subsequently sub-categorized into exponential growth, staccato growth, or indeterminate growth patterns.
Statistical Analysis
Descriptive statistics were compared between clinical groups with t-tests to test continuous variables. Biomarkers (C-reactive protein, MMP-9, and serum amyloid A) were logarithmically transformed if necessary to stabilize variance (Supplemental Figures 1a–d). Chi-square tests were used to assess categorical variables. Significance was set to ≤ 0.05 (two-sided). Unadjusted and adjusted analysis of aneurysm growth were performed using a linear mixed-effects model by restricted maximal likelihood (REML) to account for the covariance structure in the repeated measure data. Aneurysm MTD was the dependent variable. The interaction of time of imaging study following randomization with each corresponding covariate represented its effect on MTD change over time, or aneurysm growth. Adjusted growth analysis for diabetic patients was performed with potential confounders and the interaction of diabetes with doxycycline therapy. Covariates were selected if hypothesized in other literature to have a possible association with AAA diameter and growth. In addition to the presence of DM and doxycycline therapy, the model contained time between each CT scan, sex, smoking, chronic obstructive pulmonary disease (COPD), angiotensin receptor blocker (ARB), body mass index (BMI), beta blockers, and statin as prespecified variables. Given that this was a post-hoc exploratory subgroup analysis of a randomized control trial, no power calculations were conducted.
RESULTS
Study Population
Of 261 patients, 250 subjects had at least two CT scan images evaluated during the trial to allow for growth rate measurements and were included in this study. Of this group, there were 56 (22.4%) patients with diabetes and 194 (77.6%) without diabetes as self-reported. The clinical characteristics of the population at entry are listed in Table I. The diagnosis of diabetes was associated with higher BMI and increased rates of hypercholesterolemia, and coronary artery disease (CAD), and lower rates of cancer (p< 0.05). Diabetes was also associated with increased frequency of treatment for hypercholesterolemia and hypertension including treatment with statin, angiotensin-converting enzyme (ACE) inhibitor, ARB, any anti-platelet, and diuretic therapy (p< 0.05). At study entry, aneurysm MTD was not significantly different between groups (p=0.67). The circulating concentrations of the three inflammatory biomarkers were not significantly different between groups.
Table I.
Baseline characteristics: Non-diabetics and Diabetics
| Characteristic | No history of Diabetes (N=194) Mean (±SD); N(%) Median (IQR) |
History of Diabetes (N=56) Mean (±SD); N(%) Median (IQR) |
Total (N=250) Mean (±SD); N(%) Median (IQR) |
|---|---|---|---|
| Age | 71 (±7.6) | 70 (±6.5) | 71 (±7.3) |
| Sex | |||
| Male | 165 (85%) | 50 (89%) | 215 (86%) |
| Female | 29 (15%) | 6 (11%) | 35 (14%) |
| BMI** | 28.4 (±5.2) | 31.2 (±4.4) | 29 (±5.1) |
| Race | |||
| White | 176 (90.7%) | 53 (94.6) | 229 (91.6%) |
| Non-white | 18 (9.3%) | 3 (5.4%) | 21 (8.4%) |
| Smoking | |||
| Never used | 15 (7.7%) | 4 (7.1%) | 19 (7.6) |
| Current user/quit in last year | 69 (35.6%) | 15 (26.8%) | 84 (33.6%) |
| Former user | 110 (56.7%) | 37 (66.1%) | 147 (58.8%) |
| Hypercholesterolemia* | 145 (74.7%) | 51 (91.1%) | 196 (78.4%) |
| CAD* | 74 (38.1%) | 30 (53.6%) | 104 (41.6%) |
| Cancer* | 70 (36.1%) | 11 (19.6%) | 81 (32.4%) |
| COPD | 46 (23.7%) | 13 (23.2%) | 59 (23.6%) |
| Family history AAA | 39 (20.1%) | 8 (14.3%) | 47 (18.8%) |
| Atrial Fibrillation | 23 (11.9%) | 9 (16.1%) | 32 (12.8%) |
| CHF* | 11 (5.7%) | 8 (14.3%) | 19 (7.6%) |
| Medications | |||
| Statin** | 151 (77.8%) | 54 (96.4%) | 205 (82.0%) |
| Beta blocker | 95 (49.0%) | 35 (62.5%) | 130 (52%) |
| ACE inhibitor* | 61 (31.4%) | 26 (46.4%) | 87 (34.8%) |
| ARB* | 29 (15%) | 16 (28.6%) | 45 (18%) |
| CCB | 43 (22.2%) | 16 (28.6%) | 59 (23.6%) |
| Daily ASA | 130 (67%) | 45 (80.4%) | 175 (70%) |
| Other anti-platelet | 33 (17.0%) | 10 (17.9%) | 43 (17.2%) |
| Any anti-platelet* | 137 (70.6%) | 49 (87.5%) | 186 (74.4%) |
| Diuretic** | 52 (26.8%) | 29 (51.8%) | 81 (32.4%) |
| Baseline MTD (cm) | 4.30 (±0.41) | 4.32 (±0.45) | 4.30 (±0.42) |
| Males | 4.35 (±0.40) | 4.34 (±0.47) | 4.35 (±0.42) |
| Females | 3.97 (±0.32) | 4.16 (±0.26) | 4.00 (±0.32) |
| Repairs | 19 (9.8%) | 3 (5.4%) | 22 (8.8%) |
| Death | 4 (2%) | 1 (1.8%) | 5 (2%) |
| CRP (mg/L) | 2.53 (1.19-4.68) | 2.1 (1.31-3.82) | 2.34 (1.20-4.56) |
| MMP-9 (ng/mL) | 38.90 (26.53-59.82) | 34.43 (27.87-45.20) | 37.86 (27.18-56.83) |
| SAA (mg/L) | 19.15 (10.80-40.40) | 22.97 (14.04-40.66) | 20.70 (12.04-40.40) |
p<0.05
p<0.005
Interquartile range (IQR), standard deviation (SD)
Unadjusted Effect of Individual Characteristics on Maximum Transverse Diameter Growth of Small Aortic Aneurysms
In addition to diabetes mellitus, several of the baseline characteristics of AAA patients have been shown or hypothesized to modify growth of the AAA. We performed unadjusted modeling of the effect of the selected baseline characteristics on the growth of the aneurysm using a linear mixed-effects model by REML. This modeling was selected to account for the repeated measures of the data in each individual and the variable intervals between the CT images. In these unadjusted analyses, many of these factors were associated with significant modification of the growth of the AAA during N-TA3CT as shown in Table II. Reported female gender (p=0.01) and those reporting active smoking at enrollment (p =0.0001; compared to former and never smoking) were significantly associated with increased aneurysm growth. Diabetes (p<0.0001), BMI (p<0.01), ARB therapy (p<0.0001), and statin therapy (p<0.0001) were associated with decreased aneurysm growth. There was no statistically significant unadjusted effect of COPD, prior smoking, or beta-blocker therapy on the rate of AAA growth during the study.
Table II.
Unadjusted Analysis: Clinical Characteristics and Growth Rate
| Clinical Characteristic | AAA Growth Rate without Clinical Characteristic (cm/yr) | Change in Growth Rate with Clinical Characteristic (cm/yr) | Clinical Characteristic*time p-value |
|---|---|---|---|
| Randomized Doxycycline Rx | 0.19 | 0.01 | 0.43 |
| Diabetes | 0.21 | −0.06 | <0.0001 |
| Female sex | 0.21 | −0.02 | 0.01 |
| Smoking current | 0.19 | 0.05 | 0.0001 |
| Smoking former | 0.19 | −0.02 | 0.66 |
| COPD | 0.19 | 0.00 | 0.24 |
| ARB therapy | 0.20 | −0.06 | <0.0001 |
| Statin therapy | 0.22 | −0.03 | <0.0001 |
| Beta blocker therapy | 0.20 | −0.01 | 0.22 |
| BMI (per kg/m2) | −0.0019 | <0.01 | |
Unadjusted associations of clinical characteristics and the AAA growth rate in the N-TA3CT. Mean growth rates as calculated by linear regression. P-values presented for each clinical characteristic are derived from a linear mixed effects model on AAA growth. BMI is treated as a continuous variable – the effect on the AAA growth is presented as a linear coefficient.
Effect of Diabetes on Maximum Transverse Growth of Small Aortic Aneurysms
Mean AAA MTD growth in those with diabetes was 0.15 cm/yr ±0.13 and in those without diabetes was 0.21 cm/yr ± 0.14. Median growth rate for patients with diabetes was 0.12 cm/yr (IQR 0.07-0.22) and for patients not diagnosed with diabetes was 0.19 cm/yr (IQR 0.12-0.27). The distributions of the calculated growth rates are as shown in Figure 1. The distribution is shifted to the left in patients with a diagnosis of diabetes and there are fewer patients with relatively rapid growth rates.
Figure 1: Aneurysm Growth Rate Distribution.
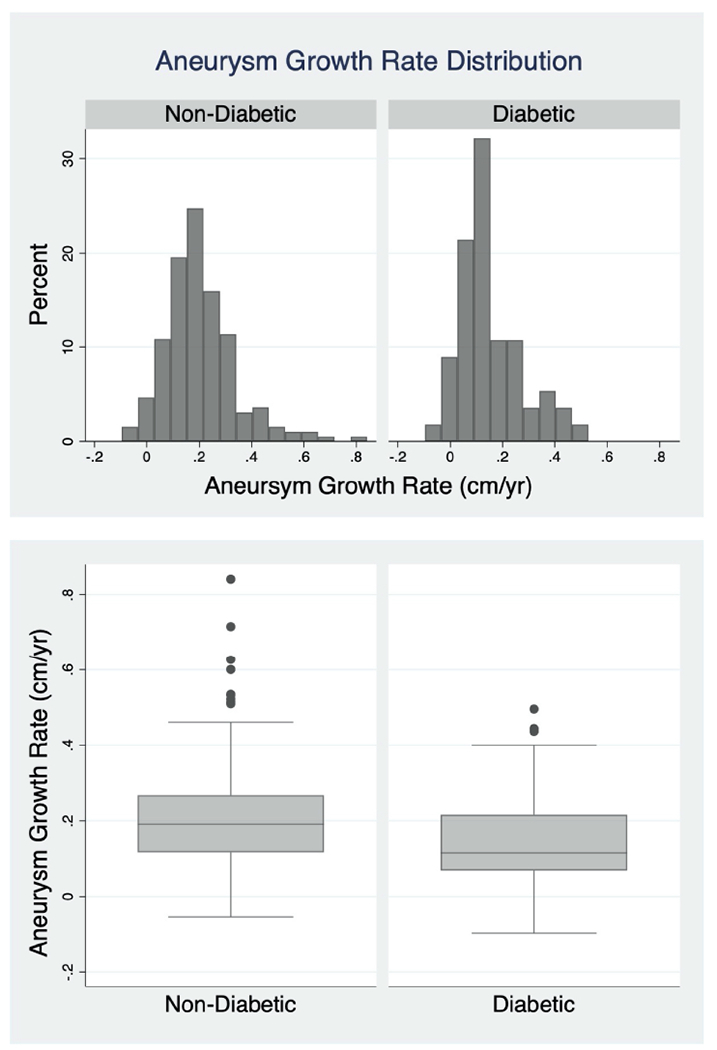
The distributions of the growth rates as calculated by linear regression are demonstrated by histogram above and the box and whisker plot below with the non-diabetic group on the left and diabetics on the right. Those with a diagnosis of diabetes exhibited a mean growth rate of 0.15 cm/yr (±0.13) and median rate of 0.12 cm/yr (IQR 0.07-0.22). Those without a diagnosis of diabetes exhibited a mean growth rate of 0.21 cm/yr (±0.14) and median rate of 0.19 cm/yr (IQR 0.12-0.27).
The effect of diabetes on growth of these small aortic aneurysms was evaluated in a adjusted model using covariates which were suspected or known to have an effect on AAA growth. The variables included in the model were sex, COPD, BMI, randomization to doxycycline treatment, smoking status, and treatment at enrollment with ARBs, beta-blockers or statins. We also included an interaction term of doxycycline with diabetes. In the adjusted analysis, a diagnosis of diabetes remained significantly associated with slower AAA growth (p<0.0001). The interaction of diabetes with doxycycline therapy on MTD was not significant (p=0.08).
Effect of Diabetes on Aneurysm Growth Patterns
Of the 261 patients, 214 had sufficient imaging for growth pattern analysis. Of this group, there were 163 without diabetes (76.2%) and 51 (23.8%) patients with diabetes. Those with diabetes exhibited significantly different growth patterns compared to those without as seen in Table III (p<0.05). The growth patterns of patients with diabetes and those without appear to have a similar small proportion of patients that are characterized as exponential (4%) or staccato (4% and 3%, respectively). Like those free of diabetes (75%), most of the patients with diabetes (53%) demonstrated a linear growth pattern. However, proportionally more of the patients with diabetes had indeterminate growth patterns (40%) compared to those without diabetes (19%).
Table III.
Effect of Diabetes on Aneurysm Growth Patterns
| Growth Pattern | Non-Diabetic (n=163) | Diabetic (n=51) | Total (n=214) |
|---|---|---|---|
| Linear | 122 (75%) | 27 (53%) | 149 (70%) |
| Indeterminate—No Growth | 11 (7%) | 10 (20%) | 21 (10%) |
| Indeterminate—Growth | 19 (12%) | 10 (20%) | 29 (14%) |
| Exponential | 6 (4%) | 2 (4%) | 8 (4%) |
| Staccato | 5 (3%) | 2 (4%) | 7 (3%) |
Differences in growth patterns between diabetic and non-diabetics groups were statistically significant on chi-square analysis (p=0.025).
Predicting the Effect of Diabetes on Growth to Clinical Threshold for Repair
We have previously demonstrated that the vast majority of the patients with aneurysms in the size range enrolled in N-TA3CT grow with a pattern that is best described as linear.13 Among the patients with diabetes, the growth patterns were similar although more patients are categorized as indeterminate and fewer categorized as linear. Under the assumption of linear growth using median growth rates for men and women in N-TA3CT, we modeled the effect of a diagnosis of diabetes in a patient with a small aortic aneurysm assuming an initial diameter of 4.3 cm (median baseline diameter of patients enrolled in N-TA3CT). In men, a patient without a diagnosis of diabetes will cross the threshold for repair (5.5 cm) in 6.7 years while a patient with diabetes will reach that threshold in approximately 10.9 years (Figure 2a). In women, patients without diabetes will reach the threshold for repair (5.0 cm) typically in 3.7 years, while those with diabetes will need repair in 5.0 years, reflecting a faster growth rate of women included in N-TA3CT as well as the lower threshold for intervention (Figure 2b).
Figure 2. Predicting Aneurysm Repair by Diabetes in Men and Women.
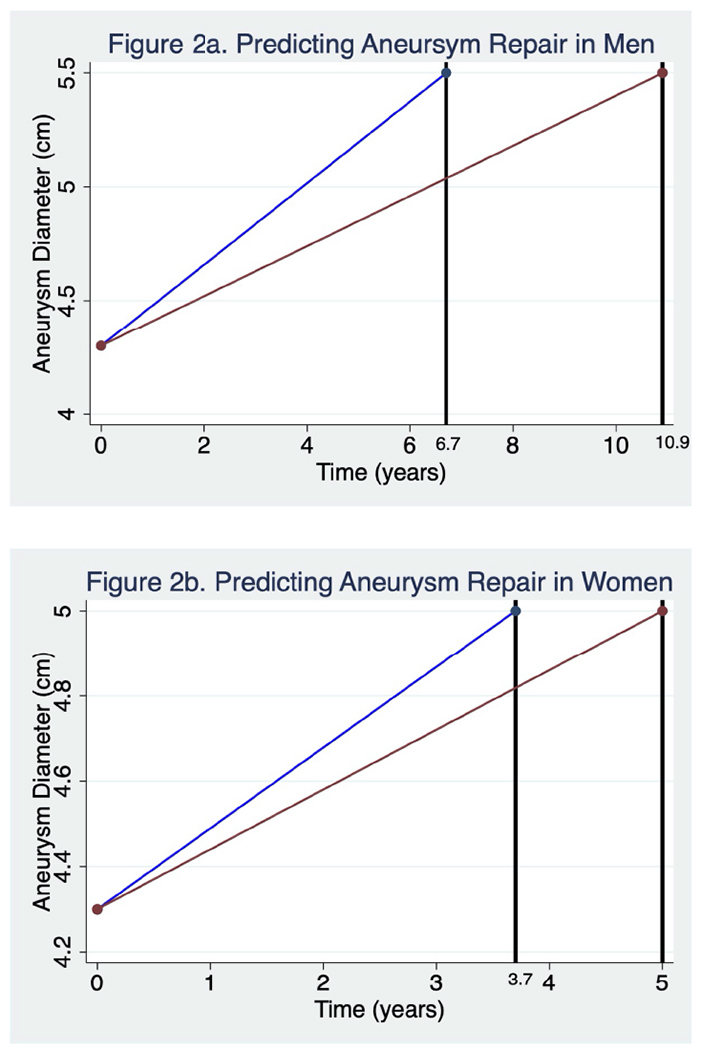
a and b. Under the assumption of linear growth, we modeled the effect of a diagnosis of diabetes in a patient with a small aortic aneurysm assuming and initial diameter of 4.3 cm (median baseline diameter of patients enrolled in N-TA3CT) applying yearly median growth rates as calculated by linear regression. Aneurysm growth in those with a diagnosis of diabetes is represented by a red line and growth in those without a diagnosis of diabetes is represented by a blue line. The vertical black lines represent the points in time at which repair thresholds are projected to be met (5.5 cm in men and 5 cm in women).
DISCUSSION
In the patients with aneurysms not large enough to be excluded from this multicenter randomized trial, a diagnosis of diabetes is associated with a 37% reduction in AAA maximal transverse diameter (MTD) median growth. The distribution of growth rates of aneurysms in patients with diabetes was distinct from the patients without diabetes, being more tightly grouped toward lower growth rates (Figure 1). None of the patients with diabetes had a growth rate of greater than 0.5 cm/yr compared to the 5% of patients free of diabetes that demonstrated this relatively fast growth rate. In patients with diabetes, the growth was dominantly linear, although there were more patients with no detectable growth (less than 0.05 cm/yr) over the course of the study which increased the proportion that had an indeterminate growth pattern.
A reduced incidence of AAA in patients with diabetes was first demonstrated by Lederle et al. Other large population studies have also shown no difference or reduced incidence of AAA.4,5,14 No studies have associated increased growth of the aneurysm with diabetes,15,16 and a few have shown slower aneurysm growth anywhere between 0.001 cm/yr and 0.2 cm/yr.7,17,18 Our study’s growth rate difference between those with and without diabetes (−0.07 cm/yr) is larger than that estimated by the RESCAN investigators in a meta-analysis of 18 longitudinal studies representing more than 15,000 patients.17 The ability to identify a difference in the relatively small N-TA3CT cohort may be attributed to the exclusive use of CT scan imaging, the central measurement techniques of the imaging core laboratory and low attrition rates of the study participants. These findings support the N-TA3CT study design assumptions that these techniques have sufficient power to detect aortic MTD growth differences of at least 40% over 2 years of follow-up.9
The patients with diabetes enrolled in N-TA3CT demonstrated more frequent concomitant co-morbidities, including comorbidities related to atherosclerotic vascular disease. Treatment for hyperglycemia and vascular comorbidities results in more drug-class therapies than the patients without diabetes and some of these therapies may independently modify AAA growth. In this cohort, we found that patients with diabetes were more likely to be treated with ARB, ACE-inhibitors, statins, anti-platelet agents, diuretics, and beta blockers than patients without diabetes. Patients with diabetes were also found to have a higher BMI as well as more frequent diagnoses of CAD, CHF, and hypercholesterolemia. In the N-TA3CT, metformin (or other hypoglycemic therapy) use was not specifically collected during the trial. Without these data, we cannot analyze the effects of DM on AAA growth in the context of variations in DM management regimens. There already exist preclinical and retrospective evaluations of large groups of AAA patients which suggest that metformin therapy may contribute to the effect of diabetes slowing AAA growth.19–22 Based on these studies, there are ongoing clinical trials which are evaluating the potential for metformin to alter AAA growth in patients without diabetes.23–25 The effect of DM on AAA growth likely has a more complex mechanism than explained by metformin alone.26 We anticipate that these findings can form the basis for further mechanistic studies of the effect of diabetes on AAA growth, including analysis of additional biomarkers in patients within N-TA3CT.6,27,28
On unadjusted analysis, several baseline factors in addition to diabetes were significantly associated with altered growth rates of AAA during the trial. This includes BMI, female sex, active smoking, and treatment with ARB or statins. These variables and others that were thought to be potentially associated with aneurysm growth were included in a mixed linear model with the primary variable of interest, the effect of diabetes on the change in diameter over time.29–31 The effect of diabetes on AAA growth remained statistically significant and robust in this adjusted model. A recent randomized control trial found no effect of ARB use on AAA growth.32 The relationship between statin therapy and AAA growth presents numerous challenges to validate particularly in the context of the known clinical benefits not related to AAA growth, and no randomized, controlled trial exists or is planned.33
The differences in growth rates observed for patients with diabetes have significant implications regarding future aneurysm management. Slower growth may result in a delay in reaching the threshold for aneurysm repair by several years. Projecting central tendency does not account for the entire distribution of growth rates in groups. Nevertheless, none of the patients with diabetes in this study had rapid aneurysm growth (> 0.5 cm/yr), whereas 5% of those without diabetes demonstrated this fast growth rate. Consideration of longer imaging intervals in patients with diabetes may warrant additional investigation. Whether these slower growth rates will have an effect on the risk of rupture has not been studied, and no ruptures of either group with AAA as enrolled in N-TA3CT were seen in 2 years of follow-up.
There are several hypotheses that may explain the finding that patients with diabetes are more resistant to aneurysm formation and growth in addition to the potential effects of metformin. Those with diabetes have been shown to exhibit stiffer arterial walls as a result of increased extracellular matrix volume as well as matrix non-enzymatic glycation.34–37 These glycated matrices are associated with resistance to MMP-mediated proteolysis.34,35 Induced hyperglycemia has been shown to reduce aneurysm growth in animal models.6 Kristenson et al. also demonstrated a significant inverse relationship between HbA1C levels and AAA growth in human subjects.28 It is also hypothesized that aortic aneurysm intraluminal thrombus in diabetes is more resistant to thrombolysis and subsequently contributes some anti-proteolytic effects while simultaneously exerting a mechano-protective effect on the adjacent aneurysmal aortic wall.36–38
This study has several limitations. The original N-TA3CT trial was not designed to compare patients with diabetes to those without. There were many differences in the characteristics of these two groups. Diabetes medication histories were not collected. Additionally, clinical hemoglobin HbA1C values were not collected, limiting this study’s ability to evaluate the effect of persistent hyperglycemia on AAA growth. Baseline inflammatory markers were not different between those with diabetes and those without, but a detailed investigation of the relationship of inflammatory markers to AAA growth rate between these groups may be worth additional investigation.
CONCLUSION
In a prospective trial of small AAA, a diagnosis of diabetes was associated with an overall reduction in aneurysm growth rate despite more severe concomitant vascular co-morbidities and similar initial sizes of aneurysms. The distribution of growth rates was generally similar to those with diabetes but lacked patients with relatively fast growth.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research:
Retrospective subgroup analysis of a clinical trial
Key findings:
Of 261 patients, 250 had sufficient imaging for analysis. There were 56 (22.4%) patients affected by diabetes and 194 (77.6%) not affected. The median aneurysm growth rate was 37 percent slower in those with diabetes (0.12 cm/yr IQR 0.07-0.22) than without (0.19 cm/yr IQR 0.12-0.27). This difference was highly significant after multiple adjustments in a mixed linear model (p<0.001)
Take home Message:
The diagnosis of diabetes was associated with an overall reduction in aneurysm growth rate despite more severe concomitant vascular co-morbidities and similar initial sizes of aneurysms. Diabetic patients’ growth rates were shifted to the left and reduced in frequency of patients with relatively fast growth.
Conflict of Interest/Funding Statement
M Nordness: Nothing to disclose
BT Baxter: Grant support from the National Institute of Aging, National Institutes of Health (NIA-NIH) (R01AG037120)
J Matsumura: Grant support from the NIH (R01AG037120), Abbott, Cook, Medtronic, Gore, and Endologix
M Terrin: Grant support from the NIH (R01AG037120), P30 AG0288747 and UL1 TR003098
K Zhang: Nothing to disclose
F Ye: Nothing to disclose
NR Webb: Grant support from the NIH (R01HL134731) and American Heart Association (SFRN33900001)
R Dalman: Nothing to disclose
J Curci: Grant support from the NIH (R01AG037120 and R01HL134731).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presentation Information: Portions of these results were presented at the 2021 Southern Association for Vascular Surgery 45 Annual Meeting January 28, 2021
References
- 1.Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52(3):539–548. [DOI] [PubMed] [Google Scholar]
- 2.Mussa FF. Screening for abdominal aortic aneurysm. J Vasc Surg. 2015;62(3):774–778. [DOI] [PubMed] [Google Scholar]
- 3.Deaths, percent of total deaths, and death rates for the 15 leading causes of death in 5-year age groups, by race, and sex: United States, 2013. https://www.cdc.gov/nchs/data/dvs/lcwk1_2015.pdf. Published 2015. Accessed May 9, 2020.
- 4.Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126(6):441–449. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA. The strange relationship between diabetes and abdominal aortic aneurysm. Eur J Vasc Endovasc Surg. 2012;43(3):254–256. [DOI] [PubMed] [Google Scholar]
- 6.Miyama N, Dua MM, Yeung JJ, Schultz GM, Asagami T, Sho E, et al. Hyperglycemia limits experimental aortic aneurysm progression. J Vasc Surg. 2010;52(4):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Rango P, Farchioni L, Fiorucci B, Lenti M. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(3):243–261. [DOI] [PubMed] [Google Scholar]
- 8.Golledge J, Cooper ME, Chai Z. Diabetes and Aortic Aneurysm. Angiology. 2016;67(6):510–512. [DOI] [PubMed] [Google Scholar]
- 9.Baxter BT, Matsumura J, Curci J, McBride R, Blackwelder WC, Liu X, et al. Non-invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial (N-TA(3)CT): Design of a Phase IIb, placebo-controlled, double-blind, randomized clinical trial of doxycycline for the reduction of growth of small abdominal aortic aneurysm. Contemporary clinical trials. 2016;48:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baxter BT, Matsumura J, Curci JA, McBride R, Larson L, Blackwelder W, et al. Effect of Doxycycline on Aneurysm Growth Among Patients With Small Infrarenal Abdominal Aortic Aneurysms: A Randomized Clinical Trial. Jama. 2020;323(20):2029–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Variability in measurement of abdominal aortic aneurysms. Abdominal Aortic Aneurysm Detection and Management Veterans Administration Cooperative Study Group. J Vasc Surg. 1995;21(6):945–952. [DOI] [PubMed] [Google Scholar]
- 12.Bhak RH, Wininger M, Johnson GR, Lederle FA, Messina LM, Ballard DJ, et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150(1):44–50. [DOI] [PubMed] [Google Scholar]
- 13.Olson SL, Wijesinha MA, Panthofer AM, Blackwelder WC, Upchurch GR Jr., Terrin ML, et al. Evaluating Growth Patterns of Abdominal Aortic Aneurysm Diameter With Serial Computed Tomography Surveillance. JAMA Surg. 2021;156(4):363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8(2):92–102. [DOI] [PubMed] [Google Scholar]
- 15.Schlösser FJ, Tangelder MJ, Verhagen HJ, van der Heijden GJ, Muhs BE, van der Graaf Y, et al. Growth predictors and prognosis of small abdominal aortic aneurysms. J Vasc Surg. 2008;47(6):1127–1133. [DOI] [PubMed] [Google Scholar]
- 16.Chang JB, Stein TA, Liu JP, Dunn ME. Risk factors associated with rapid growth of small abdominal aortic aneurysms. Surgery. 1997;121(2):117–122. [DOI] [PubMed] [Google Scholar]
- 17.Sweeting MJ, Thompson SG, Brown LC, Powell JT. Meta-analysis of individual patient data to examine factors affecting growth and rupture of small abdominal aortic aneurysms. Br J Surg. 2012;99(5):655–665. [DOI] [PubMed] [Google Scholar]
- 18.Lederle FA, Noorbaloochi S, Nugent S, Taylor BC, Grill JP, Kohler TR, et al. Multicentre study of abdominal aortic aneurysm measurement and enlargement. Br J Surg. 2015; 102(12): 1480–1487. [DOI] [PubMed] [Google Scholar]
- 19.Itoga NK, Rothenberg KA, Suarez P, Ho TV, Mell MW, Xu B, et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69(3):710–716.e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golledge J, Moxon J, Pinchbeck J, Anderson G, Rowbotham S, Jenkins J, et al. Association between metformin prescription and growth rates of abdominal aortic aneurysms. Br J Surg. 2017; 104(11): 1486–1493. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Guo J, Han X, Xue M, Wang W, Mi L, et al. Metformin represses the pathophysiology of AAA by suppressing the activation of PI3K/AKT/mTOR/autophagy pathway in ApoE−/−mice. Cell & Bioscience. 2019;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujimura N, Xiong J, Kettler EB, Xuan H, Glover KJ, Mell MW, et al. Metformin treatment status and abdominal aortic aneurysm disease progression. J Vasc Surg. 2016;64(1):46–54.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalman RL. Limiting AAA With Metformin (LIMIT) Trial. https://clinicaltrials.gov/ct2/show/NCT04500756?term=metformin&cond=Abdominal+Aortic+Aneurysm&draw=2&rank=3. Published 2021. Accessed.
- 24.Neumayer C Metformin Therapy in Non-diabetic AAA Patients. https://clinicaltrials.gov/ct2/show/NCT03507413?term=metformin&cond=Abdominal+Aortic+Aneurysm&draw=2&rank=2. Published 2018. Accessed. [Google Scholar]
- 25.Unosson J Metformin for Abdominal Aortic Aneurysm Growth Inhibition. https://clinicaltrials.gov/ct2/show/NCT04224051?term=metformin&cond=Abdominal+Aortic+Aneurysm&draw=2&rank=1. Published 2020. Accessed. [DOI] [PubMed] [Google Scholar]
- 26.Thompson A, Cooper JA, Fabricius M, Humphries SE, Ashton HA, Hafez H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J Vasc Surg. 2010;52(1):55–61.e52. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen KL, Pottegard A, Hallas J, Rasmussen LM, Lindholt JS. Metformin treatment does not affect the risk of ruptured abdominal aortic aneurysms. J Vasc Surg. 2017;66(3):768–774.e762. [DOI] [PubMed] [Google Scholar]
- 28.Kristensen KL, Dahl M, Rasmussen LM, Lindholt JS. Glycated Hemoglobin Is Associated With the Growth Rate of Abdominal Aortic Aneurysms: A Substudy From the VIVA (Viborg Vascular) Randomized Screening Trial. Arteriosclerosis, thrombosis, and vascular biology. 2017;37(4):730–736. [DOI] [PubMed] [Google Scholar]
- 29.Salata K, Syed M, Hussain MA, de Mestral C, Greco E, Mamdani M, et al. Statins Reduce Abdominal Aortic Aneurysm Growth, Rupture, and Perioperative Mortality: A Systematic Review and Meta-Analysis. Journal of the American Heart Association. 2018;7(19):e008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salata K, Syed M, Hussain MA, Eikelboom R, de Mestral C, Verma S, et al. Renin-angiotensin system blockade does not attenuate abdominal aortic aneurysm growth, rupture rate, or perioperative mortality after elective repair. J Vasc Surg. 2018;67(2):629–636.e622. [DOI] [PubMed] [Google Scholar]
- 31.Lederle FA, Johnson GR, Wilson SE, Aneurysm D, Management Veterans Affairs Cooperative S. Abdominal aortic aneurysm in women. Journal of Vascular Surgery. 2001;34(1):122–126. [DOI] [PubMed] [Google Scholar]
- 32.Morris DR, Cunningham MA, Ahimastos AA, Kingwell BA, Pappas E, Bourke M, et al. TElmisartan in the management of abDominal aortic aneurYsm (TEDY): The study protocol for a randomized controlled trial. Trials. 2015;16:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan Z, Cui H, Wu N, Zhang H. Effect of Statin Therapy on Abdominal Aortic Aneurysm Growth Rate and Mortality: A Systematic Review and Meta-analysis. Ann Vasc Surg. 2020;67:503–510. [DOI] [PubMed] [Google Scholar]
- 34.Golledge J, Karan M, Moran CS, Muller J, Clancy P, Dear AE, et al. Reduced expansion rate of abdominal aortic aneurysms in patients with diabetes may be related to aberrant monocyte-matrix interactions. Eur Heart J. 2008;29(5):665–672. [DOI] [PubMed] [Google Scholar]
- 35.Koole D, van Herwaarden JA, Schalkwijk CG, Lafeber F, Vink A, Smeets MB, et al. A potential role for glycated cross-links in abdominal aortic aneurysm disease. J Vasc Surg. 2017;65(5):1493–1503.e1493. [DOI] [PubMed] [Google Scholar]
- 36.Raffort J, Lareyre F, Clement M, Hassen-Khodja R, Chinetti G, Mallat Z. Diabetes and aortic aneurysm: current state of the art. Cardiovascular research. 2018;114(13):1702–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dattani N, Sayers RD, Bown MJ. Diabetes mellitus and abdominal aortic aneurysms: A review of the mechanisms underlying the negative relationship. Diabetes & vascular disease research. 2018;15(5):367–374. [DOI] [PubMed] [Google Scholar]
- 38.Swedenborg J, Eriksson P. The intraluminal thrombus as a source of proteolytic activity. Ann N Y Acad Sci. 2006;1085:133–138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


