Abstract
Background
The COVID-19 pandemic increased the use of broad-spectrum antibiotics due to diagnostic uncertainty, particularly in critical care. Multi-professional communication became more difficult, weakening stewardship activities.
Aim
To determine changes in bacterial co-/secondary infections and antibiotics used in COVID-19 patients in critical care, and mortality rates, between the first and second waves.
Methods
Prospective audit comparing bacterial co-/secondary infections and their treatment during the first two waves of the pandemic in a single-centre teaching hospital intensive care unit. Data on demographics, daily antibiotic use, clinical outcomes, and culture results in patients diagnosed with COVID-19 infection were collected over 11 months.
Findings
From March 9th, 2020 to September 2nd, 2020 (Wave 1), there were 156 patients and between September 3rd, 2020 and February 1st, 2021 (Wave 2) there were 235 patients with COVID-19 infection admitted to intensive care. No significant difference was seen in mortality or positive blood culture rates between the two waves. The proportion of patients receiving antimicrobial therapy (93.0% vs 81.7%; P < 0.01) and the duration of meropenem use (median (interquartile range): 5 (2–7) vs 3 (2–5) days; P = 0.01) was lower in Wave 2. However, the number of patients with respiratory isolates of Pseudomonas aeruginosa (4/156 vs 21/235; P < 0.01) and bacteraemia from a respiratory source (3/156 vs 20/235; P < 0.01) increased in Wave 2, associated with an outbreak of infection. There was no significant difference between waves with respect to isolation of other pathogens.
Conclusion
Reduced broad-spectrum antimicrobial use in the second wave of COVID-19 compared with the first wave was not associated with significant change in mortality.
Keywords: COVID-19, Bacteraemia, Pseudomonas aeruginosa, Antimicrobial stewardship, Co-infection
Introduction
The COVID-19 pandemic has placed healthcare systems under immense pressure worldwide, driving increased use of antibiotics and hence the potential for emergence of antimicrobial resistance and adverse effects [1]. Several key factors have encouraged increased broad-spectrum antibiotic use, particularly carbapenems: (i) the challenge of individualizing therapy amidst overwhelming rises in numbers of patients requiring high-dependency and intensive care; (ii) uncertainty about the contribution of bacterial co-infection or secondary infection in critically ill patients with otherwise limited treatment options; and (iii) the absence of specific biochemical or imaging tests able to reliably distinguish SARS-CoV-2 disease from bacterial infection. Overuse of antibiotics risks accelerating the emergence of carbapenem-resistant organisms [2].
In 2020–21, the UK experienced two major peaks of COVID-19 infections, the first in spring 2020 and the second (predominantly alpha B1.1.7 variant) during the winter of 2020–21. The key differences in hospital management between these two waves resulted partly from experience gained with treating this new disease and from an increased use of immunosuppressive agents, particularly dexamethasone and tocilizumab, following the findings of the RECOVERY trials [3,4]. If use of such agents were associated with an increase in secondary infections, there would be further challenges for diagnosis and antimicrobial stewardship [5,6].
We thus sought to evaluate the differences between the first two waves of COVID-19 with respect to antimicrobial antibiotic use, microbiologically proven infections, and mortality in a single-centre adult intensive care unit (ICU) during the first 11 months of the COVID-19 pandemic in the UK.
Methods
The University College London Hospital adult ICU is a general medical and surgical facility, normally operating 35 beds. Bed capacity rose to 90 during the pandemic through expansion into theatre operating rooms and other ward areas to meet demand. In both waves, clinical staff were redeployed to the ICU from other services to support surge rotas. The nurse:patient ratio fell from the usual 1:1 ratio for mechanically ventilated patients to as low as 1:3 or even 1:4. In some areas, bed spacing was reduced from 3.6 to 2 m. Equipment availability and frequency of cleaning were challenged.
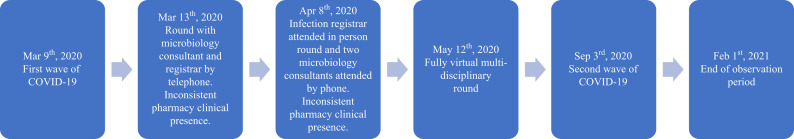
Standard antimicrobial stewardship practice for nosocomial infections, principally short courses of piperacillin–tazobactam or ceftazidime and restricted use of meropenem, had been in place on this ICU since 2000, as previously described [7]. At the start of the first wave, the ICU pharmacists were prioritized to dealing with drug shortages, liaison, and guidelines development. Antimicrobial stewardship activities by both microbiologists and pharmacists were severely limited. At the start of the first wave (Figure 1 ), senior microbiologist input changed from an in-person ward round to telephone contact. By the peak, the intense activity and need for removal of personal protective equipment before joining even a telephone conference made multi-disciplinary meetings difficult. An infectious disease or microbiology specialist trainee then attended rounds in person and two consultant microbiologists joined by telephone. Later in the first wave (May 12th, 2020), a fully virtual multi-disciplinary round was started, improving consistency and communication for stewardship and infection control purposes; this continued throughout the second wave. The virtual rounds included ICU consultants, nurses, pharmacists, physiotherapists, dieticians, psychologists, palliative care physicians, and family liaison officers involved in each patient's care.
Figure 1.
Timeline for microbiology and pharmacy liaison with critical care ward rounds.
The transition from first to second wave was taken as September 3rd, 2020 which corresponded to a short period of no COVID-19 infected admissions. In Wave 2, an enhanced clinical pharmacy team advised on individualized patient dosing based on pharmacokinetic–pharmacodynamic principles. In contrast to Wave 1, administration of meropenem was generally discouraged and its use was de-escalated or stopped as soon as clinically indicated.
Patient demographics, daily antibiotic usage, microbiology culture results and clinical outcomes were recorded prospectively as part of routine care. Missing results were found retrospectively during audit using hospital electronic healthcare records. Bronchoalveolar lavage was performed rarely due to concerns regarding aerosolization. From hospital electronic health record and pathology systems, we collected data on all patients with a clinical and/or virologically confirmed diagnosis of COVID-19 admitted to the adult ICU between September 3rd, 2020 and February 1st, 2021. As we report data as part of service evaluation, ethics committee permission was not sought. Patients were followed to discharge from ICU.
Individual patient-level data on demographics (age, sex, date of ICU admission), use of antibiotics (generic name, days of treatment per 100 ICU adjusted patient-days (DOT/1000 ICU APD)), and treatment outcomes (death in or discharge from ICU) were recorded, as well as positive cultures. The source of bacteraemia was determined by the clinical assessment of the microbiology and ICU teams based on the clinical presentation, organism isolated, and management instigated based on positive blood culture. Contaminants were those that were thought not clinically significant at the time and did not require a clinical intervention. Co-infections were defined as those diagnosed within two days of admission to ICU, whereas later infections were considered secondary. The effects of therapy for COVID-19 on bacteraemia and need for treatment have been reported separately [6].
Continuous data were treated as non-parametric and reported as median (interquartile range), with distributions compared using the Wilcoxon rank-sum test with continuity correction. Proportions were compared with Fisher's exact or Pearson's χ2-test with Yates' continuity correction as appropriate. P < 0.05 was the threshold for statistical significance. All statistical analyses and data visualization were performed using R version 3.6.0.
Results
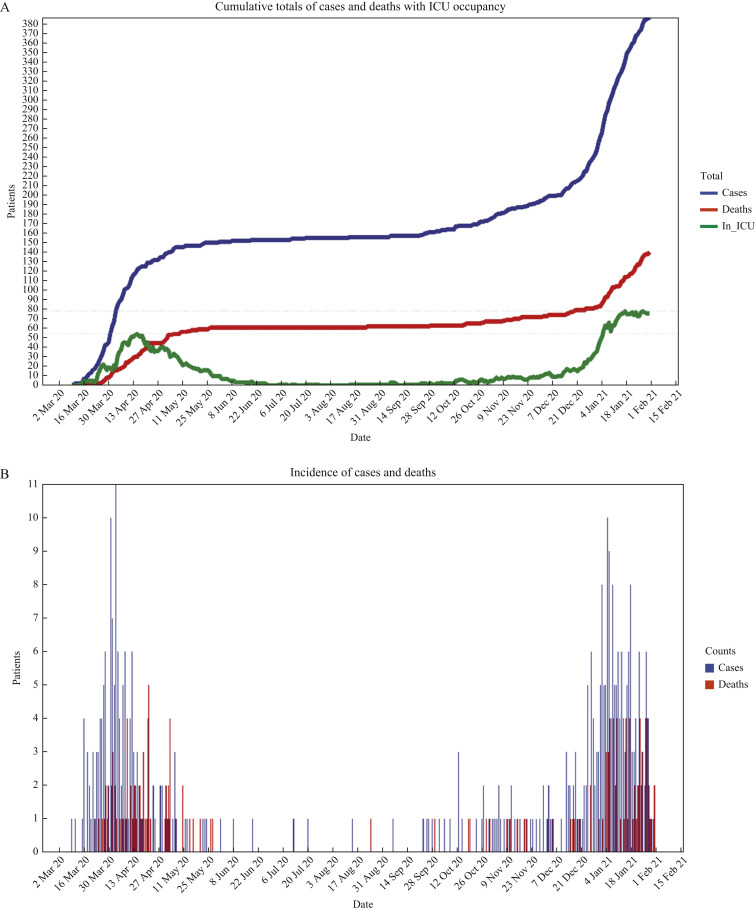
Our adult ICU admitted a total of 391 COVID-19 patients between March 9th, 2020 and February 1st, 2021: 156 in Wave 1 (March 9th to September 2nd, 2020) and 235 patients in Wave 2 (September 3rd, 2020 to February 1st, 2021). ICU COVID-19 occupancy reached a peak of 54 in Wave 1 (≥50 from April 12th to 18th, 2020) and 78 in Wave 2 (≥50 from January 4th, 2021 to February 1st, 2021) (Figure 2 A, B). Surge arrangements were in place until June 2020 during Wave 1 and until March 2021 during Wave 2.
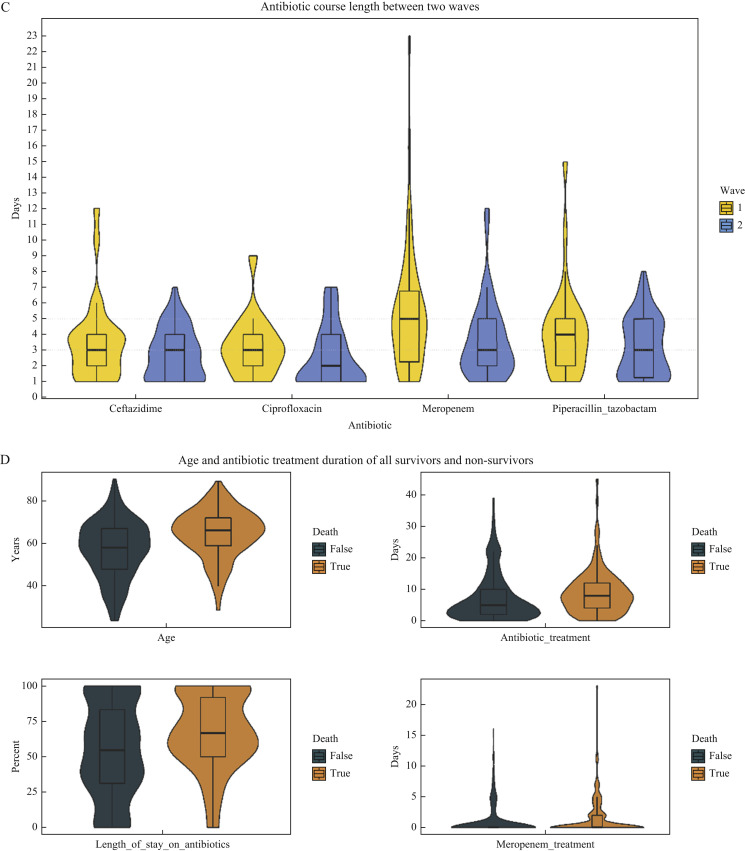
Figure 2.
(A) Cumulative total of COVID-19 patients and deaths in intensive care unit (ICU) along with daily total of COVID-19 patients in ICU over the study period. (B) Daily number of COVID-19 admissions and deaths in ICU over the study period. (C) Days of treatment per patient with most commonly used antipseudomonal antibiotics during the first two pandemic waves: violin plots of probability distribution with box plots showing median and interquartile range. (D) Age, treatment days on any antibiotic, percentage of length of stay on any antibiotic, and treatment days on meropenem of COVID-19 patients who survived (death = FALSE) or died (death = TRUE) during ICU stay: violin plots of probability distribution with box plots showing median and interquartile range.
Patient age was similar across both waves (Table I ). There was a higher proportion of females in Wave 2 (35.7% vs 25.6%; P < 0.05). The median length of stay remained consistent at 11 (IQR: 5–18) days. There was no significant difference in ICU mortality rates between waves (39.7% Wave 1, 33.2% Wave 2; P = 0.22) (Table I).
Table I.
Patient demographics, clinical outcomes, blood culture isolates, and antimicrobial use in two COVID-19 waves
| Variable | Overall (N = 391) | Wave 1 (N = 156) | Wave 2 (N = 235) | P-value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | ||||
| Median (IQR) | 61.0 (52.0, 69.0) | 60.5 (49.0, 67.0) | 61.0 (53.5, 70.0) | 0.07 |
| Sex | <0.05 | |||
| Male (%) | 267 (68.3) | 116 (74.4) | 151 (64.3) | |
| Female (%) | 124 (31.7) | 40 (25.6) | 84 (35.7) | |
| Clinical outcomes | ||||
| Death | 0.22 | |||
| Yes (%) | 140 (35.8) | 62 (39.7) | 78 (33.2) | |
| No (%) | 251 (64.2) | 94 (60.3) | 157 (66.8) | |
| Length of stay (days per patient) | ||||
| Median (IQR) | 11 (5.3, 18.0) | 10 (4.0, 21.0) | 11 (6.0, 16.0) | 0.62 |
| Antibiotic use | ||||
| Individual patient use (days) | ||||
| Median (IQR) | 6 (2.0, 11.0) | 6 (2.0, 12.0) | 6 (3.0, 10.0) | 0.66 |
| Median % of length of say (IQR) | 61.3 (37.5, 85.3) | 62.5 (40.0, 93.8) | 60.2 (33.3, 82.2) | 0.34 |
| Did not receive antibiotic (%) | 54 (13.8) | 11 (7.0) | 43 (18.3) | <0.01 |
| Blood culture isolates | ||||
| Total patients (%) | 119 (30.4) with 240 samples | 43 (27.6) with 88 samples | 76 (32.3) with 152 samples | 0.37 |
| Sources (patients) | ||||
| Clinically significant (%) | 76 (19.4) with 157 samples | 28 (17.9) with 61 samples | 48 (20.4) with 96 samples | 0.63 |
| Line (%) | 38 (9.7) with 80 samples | 18 (11.5) with 39 samples | 20 (8.5) with 41 samples | 0.41 |
| Chest (%) | 23 (5.9) with 33 samples | 3 (1.9) with 5 samples | 20 (8.5) with 28 samples | 0.01 |
| Multiple/unknown (%) | 21 (5.4) with 32 samples | 7 (4.5) with 9 samples | 14 (6.0) with 23 samples | 0.69 |
| Urine (%) | 3 (0.8) with 5 samples | 2 (1.3) with 4 samples | 1 (0.4) with 1 sample | 0.57 |
| Gastrointestinal (%) | 2 (0.5) with 4 samples | 2 (1.3) with 4 samples | 0 | 0.16 |
| Other (%) | 2 (0.5) with 3 samples | 0 | 2 (0.9) with 3 samples | 0.52 |
| Contaminant (%) | 63 (16.1) with 83 samples | 21 (13.5) with 27 samples | 42 (17.9) with 56 samples | 0.31 |
| Organisms (patients) | ||||
| Bacteria: Gram +ve (%) | 91 (23.3) with 155 samples | 33 (21.1) with 56 samples | 58 (24.7) with 99 samples | 0.49 |
| Coagulase-negative saphylococci (%) | 72 (18.4) with 112 samples | 26 (16.7) with 44 samples | 46 (19.6) with 68 samples | 0.55 |
| Staphylococcus aureus/argenteus (%) | 8 (2.0) with 12 samples | 0 | 8 (3.4) with 12 samples | 0.02 |
| Streptococcus spp. (excluding group D) (%) | 6 (1.5) with 7 samples | 0 | 6 (2.6) with 7 samples | 0.08 |
| Enterococcus spp. (%) | 14 (3.6) with 17 samples | 10 (6.4) with 12 samples | 4 (1.7) with 5 samples | 0.02 |
| Other (Corynebacterium spp., Dolosigranulum pigrum) (%) | 4 (1.0) with 7 samples | 0 | 4 (1.7) with 7 samples | 0.15 |
| Bacteria: Gram –ve (%) | 43 (11.0) with 81 samples | 15 (9.6) with 30 samples | 28 (11.9) with 51 samples | 0.58 |
| Enterobacterales (Citrobacter spp., Escherichia coli, Enterobacter spp., Klebsiella spp., Serratia spp.) (%) | 31 (7.9) with 55 samples | 13 (8.3) with 25 samples | 18 (7.7) with 30 samples | 0.96 |
| Pseudomonas aeruginosa (%) | 11 (2.8) with 15 samples | 3 (1.9) with 4 samples | 8 (3.4) with 11 samples | 0.54 |
| Environmental (Achromobacter xylosoxidans, Stenotrophomonas maltophilia) (%) | 3 (0.8) with 6 samples | 0 | 3 (1.3) with 6 samples | 0.28 |
| Acinetobacter baumannii (%) | 2 (0.5) with 3 samples | 0 | 2 (0.9) with 3 samples | 0.52 |
| Anaerobes (Fusobacterium nucleatum, Parabacteroides distasonis) (%) | 2 (0.5) with 2 samples | 1 (0.6) with 1 sample | 1 (0.4) with 1 sample | 1 |
| Fungi | ||||
| Yeast (%) | 4 (1.0) with 4 samples | 2 (1.3) with 2 samples | 2 (0.9) with 2 samples | 1 |
| Candida spp. (%) | 4 (1.0) with 4 samples | 2 (1.3) with 2 samples | 2 (0.9) with 2 samples | 1 |
IQR, interquartile range.
Total antibiotic duration of 6 (range: 2–11) days per patient did not differ between waves (P = 0.66) with at least one antibiotic given on 571 DOT/1000 ICU APD (Table II ). More patients were given no antibiotic during their ICU stay in Wave 2 (Table I). Short courses of antipseudomonal antibiotics, with median durations of antibiotic between 2 and 5 days during ICU stay, were used in both waves (Figure 2). Duration of meropenem use was significantly lower in Wave 2 (median: 3 (IQR: 2–5) vs 5 (2–7) days; P = 0.01) (Figure 2C), with meropenem given on 55 compared with 129 DOT/1000 ICU APD (P < 0.01). As a result, ceftazidime use unit-wide was significantly higher in Wave 2 than in Wave 1 (110 vs 69 DOT/1000 ICU APD, respectively) (P < 0.01). Co-amoxiclav and piperacillin–tazobactam use fell significantly in the second wave (P < 0.01). Clarithromycin was not given empirically in the second wave (Table II) as mycoplasma was not found to be causing pneumonia and there were false-positive serological tests in COVID-19-infected patients. Caspofungin use fell in the second wave (P < 0.01) but the use of other antifungals remained stable (Table II). The use of aciclovir fell after remdesivir was introduced for treatment of COVID-19 on June 26th, 2020.
Table II.
Days of treatment per 1000 intensive care-adjusted patient-days
| Antimicrobial | Total | 1st wave | 2nd wave |
|---|---|---|---|
| Meropenem | 86.73 | 129.55 | 55.33 |
| Ceftazidime | 93.08 | 69.55 | 110.33 |
| Cephalosporin/β-lactamase inhibitors | 1.92 | 0.00 | 3.33 |
| Other cephalosporins | 26.92 | 26.82 | 27.00 |
| Co-amoxiclav | 31.35 | 45.45 | 21.00 |
| Piperacillin–tazobactam | 137.50 | 150.00 | 128.33 |
| Other penicillins | 25.77 | 28.18 | 24.00 |
| Glycopeptides | 127.12 | 127.27 | 127.00 |
| Aminoglycosides | 22.12 | 21.36 | 22.67 |
| Clarithromycin | 26.92 | 52.73 | 8.00 |
| Erythromycin | 9.81 | 8.64 | 10.67 |
| Ciprofloxacin | 87.31 | 82.73 | 90.67 |
| Other antibacterials | 44.81 | 45.91 | 44.00 |
| Aciclovir | 53.65 | 80.45 | 34.00 |
| Remdesivir | 33.85 | 14.09 | 48.33 |
| Other antivirals | 8.46 | 3.64 | 12.00 |
| Caspofungin | 32.69 | 49.09 | 20.67 |
| Fluconazole | 7.69 | 9.55 | 6.33 |
| Liposomal amphotericin | 8.65 | 10.91 | 7.00 |
| Other antifungals | 5.77 | 6.23 | 5.43 |
| No anti-infective | 439.23 | 450.00 | 431.33 |
Whereas there was a significant increase in the number of positive blood cultures per 100 sets taken from Wave 1 to Wave 2 (16.7 vs 23.2; P < 0.01), there was no significant difference in the number of positive blood cultures, all bloodstream infections (positive blood cultures of clinical significance), or secondary bloodstream infections per 1000 patient-days (Table I). Only one significant positive blood culture in Wave 1 and eight from four patients in Wave 2 were co-infections (<48 h of admission). The proportion of patients with positive blood cultures (119/391, 30.4%), whether of clinical significance (76/391, 19.4%) or contaminants (63/391, 16.1%), was comparable between waves, with central venous catheters the most frequent source attributed (Table I). However, there was a significant increase in bacteraemia associated with pneumonia in Wave 2 (8.5% vs 1.9%, P = 0.01). All pneumonia-related bacteraemia in Wave 1 (3/3, 100%) and most in Wave 2 (14/20, 70%) were caused by Gram-negative organisms. There was no significant difference in Gram-negative bacteraemia types overall between the two waves, the most common isolates being Enterobacterales and Pseudomonas aeruginosa (Table I). Escherichia coli was found in only four patients compared with eight with Klebsiella pneumoniae and nine K. aerogenes. Five patients in one week in January 2021 developed pseudomonas bacteraemia in an outbreak which continued after February 1st, three also having sputum isolates. These concomitant infections accounted for 18 patient-days of antipseudomonal antibiotic treatment. However, the isolates were not typed or sequenced. Only five patients overall had P. aeruginosa isolated from both sputum and blood. Serratia spp. bacteraemia was identified in two patients in Wave 1 and six in Wave 2. There was a cluster of Staphylococcus aureus pneumonia (four isolates in four patients) in Wave 2, of which two isolates were found to be indistinguishable on multi-locus sequence typing. The only blood isolate of Streptococcus pneumoniae was in a patient with pneumonia co-infection in Wave 2. Only four patients had candidaemia and one had a respiratory isolate of Aspergillus sp. in the whole period (Tables II, III ). Elevated β-d-glucan was detected in one patient in the first wave and eight in the second.
Table III.
Pathogens isolated from sites other than blood in each wave (including polymicrobial)
| Site | Pathogen | No. of patients Apr–Aug 2020 (patient-days 2372) (N = 156) |
No. of patients Sep 2020–Jan 2021 (patient-days 3008) (N = 235) |
|---|---|---|---|
| Respiratory | Staphylococcus aureus | 12 | 15 |
| Other Gram positives | 1 | 2 | |
| Klebsiella pneumoniae | 5 | 7 | |
| Other Klebsiella spp. | 3 | 4 | |
| Pseudomonas aeruginosa | 4a | 21a | |
| Haemophilus influenzae | 3 | 7 | |
| Stenotrophomonas maltophilia | 0 | 5 | |
| Serratia marcescens | 1 | 6 | |
| Other Gram negatives | 8 | 8 | |
| Aspergillus sp. | 1 | 0 | |
| Candida sp. | 2 | 1 | |
| Urinary | Candida albicans | 8 | 14 |
| Other Candida sp. | 2 | 7 | |
| Enterococcus sp. | 0 | 9 | |
| Escherichia coli | 2 | 13 | |
| Klebsiella pneumoniae | 2 | 2 | |
| Other Klebsiella spp. | 0 | 4 | |
| Pseudomonas aeruginosa | 1 | 4 | |
| Wound | Staphylococcus aureus | 1 | 3 |
| Other Gram positives | 1 | 4 | |
| Pseudomonas aeruginosa | 0 | 5 | |
| Other Gram negatives | 2 | 2 | |
| Candida albicans | 1 | 3 |
χ2 = 5.3, P < 0.03.
Resistance rates were low. There were no bloodstream isolates of carbapenemase-producing organisms or meticillin-resistant S. aureus (MRSA). Vancomycin-resistant Enterococcus faecium (VRE) was isolated from only two patients and was thought to be line-associated. All Candida spp. isolates were susceptible to fluconazole. In Gram-negative isolates from blood, resistance to ceftazidime was reported in four patients (five occasions) in Wave 1 and three patients (four occasions) in Wave 2 (1.7 and 1.0 per 1000 patient days). None had been treated with ceftazidime. Resistance to piperacillin–tazobactam was reported in four patients (six occasions) during Wave 1 and four patients (five occasions) during Wave 2 (1.7 and 1.3 per 1000 patient-days, respectively). Resistance to ciprofloxacin was reported in one patient in Wave 1 and two in Wave 2 (0.4 and 0.7 per 1000 patient-days, respectively). Apart from a single isolate with meropenem resistance, all P. aeruginosa isolates were susceptible to the antibiotics used.
Of 119 patients with positive blood cultures, the spectrum of antibiotic regimen was increased in 15 (12.6%) patients and reduced (or antibiotics stopped) in 16 (13.4%) patients, when preliminary results were available on the day after the blood culture was taken. Another three (2.5%) patients had an increase in antimicrobial spectrum and two reduced (or stopped antibiotic) on the second day after the blood culture was taken, when susceptibility information was available. Treatment was changed within 24 h of the blood culture from piperacillin–tazobactam to meropenem on six occasions, to ciprofloxacin on two, and to temocillin on one, either after clinical deterioration or previous microbiological results.
Bacterial pathogens at sites other than blood were similar in both waves (Table III) but the number of patients from whom P. aeruginosa was isolated in respiratory secretions increased in the second wave (4/156 vs 21/235; P < 0.01), as with bacteraemia with a respiratory source (3/156 vs 20/235; P < 0.01) (Table I).
In the year prior to the COVID-19 pandemic, critical care patients in our unit were predominantly haematology, oncology, acute medical and surgical, in whom E. coli was the most frequent cause of Gram-negative bacteraemia (Table IV ). The reported rate of bacteraemia was lower than during the pandemic. The occurrence of resistant Gram-negative bacteraemia was 1.48, 0.83, and 2.2 per 1000 ICU patient-days for ceftazidime, piperacillin–tazobactam and ciprofloxacin, respectively [8]. There were no cases of MRSA and only one carbapenemase-producing organism causing bacteraemia. In the year prior to the onset of the first wave, 169 patients were treated with meropenem compared with 234 during the pandemic (March 2020 to January 2021).
Table IV.
Comparison of rates of bacteraemia per 1000 intensive care-adjusted patient-days before and during the pandemic
| Blood isolate species | Pre-pandemic |
COVID-19 patients, Apr 2020–Jan 2021 |
||||
|---|---|---|---|---|---|---|
| Apr 2019–Mar 2020 (10,791 patient-days) |
Apr–Aug 2020 (2372 patient-days) |
Sep 2020–Jan 2021 (3008 patient-days) |
||||
| No. of patients | BSIs per 1000 patient-days | No. of patients | BSIs per 1000 patient-days | No. of patients | BSIs per 1000 patient-days | |
| Total | 84 | 7.78 | 43 | 18.12 | 76 | 25.27 |
| Coagulase-negative staphylococci | 57 | 5.28 | 26 | 10.96 | 42 | 13.97 |
| Candida albicans | 2 | 0.18 | 2 | 0.84 | 2 | 0.66 |
| Enterobacter cloacae | 2 | 0.18 | 0 | 0 | 1 | 0.33 |
| Enterococcus faecium | 16 | 1.48 | 7 | 2.95 | 1 | 0.33 |
| Escherichia coli | 26 | 2.41 | 2 | 0.84 | 2 | 0.66 |
| Klebsiella pneumoniae | 9 | 0.83 | 3 | 1.26 | 3 | 1.00 |
| Pseudomonas aeruginosa | 8 | 0.74 | 3 | 1.26 | 8 | 2.66 |
| Staphylococcus aureus | 8 | 0.74 | 0 | 0 | 8 | 3.37 |
BSI, bloodstream infection.
Discussion
The COVID-19 pandemic represented a major challenge in providing safe and effective antimicrobial treatment in intensive care. Empiric use of antibiotic greatly exceeded use for microbiologically proven infections. Experience of treating this new disease and its complications improved markedly between the onset of the first and second waves. A major strength of our work is the granularity of data on microbiological culture and antimicrobial use in critically ill patients with COVID-19 across two waves.
At the start of the pandemic, a rapid increase in patient numbers and potential infections challenged antibiotic stewardship. Many clinicians, predominantly anaesthetists, redeployed from other areas of the hospital were not familiar with standard antimicrobial practice in the ICU. In common with other centres, the significant rise in patient load reduced compliance with infection prevention and control practice as well as stewardship [9,10]. However, the overall rate of secondary bacteraemia was low and mostly line-related, possibly reflecting the effect of staffing pressures on vascular catheter care.
In the second wave, reorganization of microbiology and pharmacy staffing and virtual multi-disciplinary rounds for all COVID-19 ICU surge locations, as well as increasing confidence in treating these patients, facilitated reduced use of meropenem in favour of alternatives. There was no significant difference between waves in mortality rate nor in incidence of Gram-negative bacteraemia. The use of ceftazidime rose and piperacillin–tazobactam fell but there was no significant change in use of ciprofloxacin. An outbreak of pseudomonas bacteraemia in the second wave was probably related to the pressure of patient numbers on infection control and suspension of work to eradicate pseudomonas from water supplies. Despite increasing awareness of the possibility of aspergillus infection, use of caspofungin, usually given empirically, fell [11].
Steroids were widely used in both waves, but tocilizumab was introduced during the second [6]. While tocilizumab and earlier use of steroids are immunosuppressive, they may promote more rapid recovery and reduced need for mechanical ventilation. This could, in turn, reduce the risk of secondary infection. The absence of individual patient-level data on comorbidities and other treatments including mechanical ventilation in our cohort limits interpretation. However, there was no significant difference in the length of ICU stay between the two waves and neither dexamethasone nor tocilizumab appeared to increase the risk of bacteraemia in this cohort of patients [6].
In our study, clinically significant isolates were found in 19.4% across the two waves, Klebsiella spp. and enterococci being the most common. Among 48,902 patients admitted to hospital in a multi-centre UK study in the first wave, 8.1% of 6157 blood cultures were positive, S. aureus and E.coli being the most common organisms [12]. In a study of UK critical care units, S. aureus and S. pneumoniae were the most common causes of co-infection (<48 h of admission), though K. pneumoniae, E. coli, and Pseudomonas aeruginosa predominated for nosocomial secondary infections [13]. Mortality was more frequent in those with bacterial infections. A retrospective study of bacteraemia in hospitalized COVID-19 patients showed low rates of Enterobacterales and high rates of coagulase-negative staphylococci in the first wave compared with pre-pandemic rates [14]. Less abdominal surgery and use of unfamiliar protective equipment were cited as possible causes. Patients admitted to critical care were much more likely to have bacteraemia and/or positive respiratory cultures than ward-level patients. Microbiologically confirmed co-/secondary bacterial infections in COVID-19 have been reported to occur in only 1% of hospital patients but case fatality rates are higher than in those without co-infection, when adjusted for age and sex [15].
Reassuringly, there were few multi-resistant bacterial isolates from blood and no significant differences between waves. In a Greek ICU, where resistant rates were high prior to COVID-19, extensively drug-resistant Acinetobacter baumannii and K. pneumoniae bacteraemia were reported in 14% and 8% of COVID-19 patients, respectively [16].
Over-prescription of antimicrobials in patients with COVID-19 infection has been a widespread problem internationally, involving 80–100% of patients admitted with COVID-19 infection, particularly in critical care units [17]. Antimicrobial stewardship has been affected adversely by the COVID-19 pandemic. In a UK survey, 65% of respondents thought that audit, quality improvement, education and multi-disciplinary meetings had been suboptimal [18]. Together with the impact on manpower, there has been a major pressure on the emergence and spread of resistance. In hospitalized patients in Korea, multidrug-resistant organisms were found in 8.6% of patients and were more common in those receiving treatment with steroids [19]. In a point prevalence survey in Scotland, one-third of 820 hospitalized patients with COVID-19 received an antibiotic for respiratory infection on the day of the survey. Around 60% of prescriptions followed local antimicrobial stewardship guidelines, with 12 of the 16 courses of mero-penem being used in critical care [20]. In a retrospectively selected sample in one hospital in the first wave, compliance with a specific local antimicrobial stewardship guideline was assessed [21]. Courses on general wards were longer than necessary even though co-infection was rare. Prescriptions in ICU were more frequently changed than on general wards and patients were less likely to have had antibiotics on admission. Overall, antibiotics could have been stopped in a quarter of patients who showed classic COVID-19 presentation.
There are limitations to our study. These are results from a single centre and may not be generalizable to other hospitals. Although demographic and antibiotic use data were collected prospectively, some microbiological data were collected retrospectively. The data preceding the pandemic were part of a national surveillance scheme (ICCQIP) and relate to a different patient population in the ICU, notably a high proportion of haematology/oncology patients [7]. The data were not adjusted for confounders such as patient comorbidities and duration of mechanical ventilation. No bacterial pathogens were isolated in many patients treated with antibiotics. Days of antibiotic use have been collected rather than dose and weight, but these are likely to be more reflective of usage than defined daily doses, which were different from local dose regimens in most cases. Although duration of central venous catheter placement was collected routinely prior to the pandemic, this was stopped during each wave as the result of staff pressures.
Infection prevention and control knowledge and management of COVID-19 changed throughout the pandemic. National personal protective equipment (PPE) guidance from Public Health England changed. SARS-CoV-2 was initially categorized as High Consequence Infectious Disease but then downgraded on March 19th, 2020 [22]. Education by the infection prevention team on safe donning and doffing of HCID PPE included double gloving and not exposing skin. Protection focused on self-protection rather than infection prevention and control practice. Healthcare workers were challenged with new guidelines and practices to protect themselves. When continued glove use was no longer recommended, this embedded practice was difficult to change, and reduced hand hygiene contributed to the risk of transmission. Others have found a decline in hand hygiene compliance during this time [23].
Antibiotic use in critical care is often based on clinical concern about a deteriorating patient with non-specific signs rather than microbiological evidence for infection. Reducing broad-spectrum antimicrobial use in the second wave of COVID-19 compared with the first wave did not significantly affect mortality in critically ill patients. At times of severe pressure on the frontline medical and nursing staff, microbiological and infection control liaison needs to be robust to ensure safe and effective treatment.
Acknowledgement
We thank the Infection Prevention and Intensive Care teams for their help.
Conflict of interest statement
A.P.R.W. is a member of a Drug Safety Monitoring Board for biologics for Roche. D.B. and M.S. have received honoraria for providing educational sessions on behalf of bioMérieux. M.S. is, or has recently sat, on advisory boards to Abbott, Biotest, Deepull, Roche Diagnostics, Pfizer, Safeguard Biosystems. No other authors declared any conflicts of interest.
Funding sources
A.P.R.W. and D.B. were supported in part by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
References
- 1.Angell K.E., Lawler J.V., Hewlett A.L., Rupp M.E., Bergman S.J., Van Schooneveld T.C., et al. Antibacterial use in the age of SARS-CoV-2. JAC Antimicrob Resist. 2021;3:dlab073. doi: 10.1093/jacamr/dlab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson A.P.R. Sparing carbapenem usage. J Antimicrob Chemother. 2017;72:2410–2417. doi: 10.1093/jac/dkx181. [DOI] [PubMed] [Google Scholar]
- 3.RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooistra E.J., van Berkel M., van Kempen N.F., van Latum C.R.M., Bruse N., Frenzel T., et al. Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit Care. 2021;25:281. doi: 10.1186/s13054-021-03717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMillan T., Jones C., O’Connor C.J., Nolan D., Chan X.H.S., Ellis J., et al. Risk factors associated with bloodstream infections among critically ill patients with COVID-19. J Infect. 2021;83e1–e3 doi: 10.1016/j.inf.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Santis V., Gresoiu M., Corona A., Wilson A.P.R., Singer M. Bacteraemia incidence, causative organisms and resistance patterns, antibiotic strategies and outcomes in a single university hospital ICU: continuing improvement between 2000 and 2013. J Antimicrob Chemother. 2015;70:273–278. doi: 10.1093/jac/dku338. [DOI] [PubMed] [Google Scholar]
- 8.Gerver S.M., Mihalkova M., Bion J.F., Wilson A.P.R., Chudasama D., Johnson A.P., et al. Surveillance of bloodstream infections in intensive care units in England. May 2016–April 2017: epidemiology and ecology. J Hosp Infect. 2020;106:1–9. doi: 10.1016/j.jhin.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Falcone M., Tiseo G., Giordano C., Leonildi A., Menichini M., Vecchione A., et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: a prospective observational study. J Antimicrob Chemother. 2021;76:1078–1084. doi: 10.1093/jac/dkaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo A., Gavaruzzi G., Borrazzo C., Olivia A., Alessandri F., Magnanimi E., et al. Multidrug-resistant Acinetobacter baumannii infections in COVID-19 patients hospitalised in intensive care unit. Infection. 2022;50:83–92. doi: 10.1007/s15010-021-01643-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kariyawasam R.M., Dingle T.C., Kula B.E., Vandermeer B., Sligl W.I., Schwartz I.S. Defining COVID-19 associated pulmonary aspergillosis: systematic review and meta-analysis. Clin Microbiol Infect. 2022 Feb 9 doi: 10.1016/j.cmi.2022.01.027. S1198-743X(22)00051-9 [online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell C.D., Fairfield C.J., Drake T.M., Turtle L., Seaton R.A., Wootton D.G., et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. Lancet Microbe. 2021;2:e354–e365. doi: 10.1016/S2666-5247(21)00090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baskaran V., Lawrence H., Lansbury L.E., Webb K., Safavi S., Zainuddin N., et al. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J Med Microbiol. 2021;70 doi: 10.1099/jmm.0.001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denny S., Rawson T.M., Hart P., Satta G., Abdulaal A., Hughes S., et al. Bactaeraemia variation during the COVID-19 pandemic;a multi-centre UK secondary care ecological analysis. BMC Infect Dis. 2021;21:556. doi: 10.1186/s12879-021-06159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerver S.M., Guy R., Wilson K., Thelwall S., Nsonwu O., Rooney G., et al. National surveillance of bacterial and fungal co- and secondary infection in COVID-19 patients in England – lessons from the first wave. Clin Microbiol Infect. 2021;27:1658–1665. doi: 10.1016/j.cmi.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokkoris S., Papchatzakis I., Gavrielatou E., Ntaidou T., Ischaki E., Malchias S., et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J Hosp Infect. 2021;107:95–97. doi: 10.1016/j.jhin.2020.11.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ansari S., Hays J.P., Kemp A., Okechukwu R., Murugaiyan J., Deo-gratias Ekwanzala M., et al. The potential impact of the COVID-19 pandemic on global antimicrobial and biocide resistance: an AMR Insights global perspective. JAC Antimicrob Resist. 2021;3:diab038. doi: 10.1093/jacamr/dlab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashiru-Oredopu D., Kerr F., Hughes S., Urch J., Lanzman M., Yau T., et al. Assessing the impact of COVID-19 on antimicrobial stewardship activities/programs in the United Kingdom. Antibiotics (Basel) 2021;10:110. doi: 10.3390/antibiotics10020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Son H., Kim T., Lee E., Park S.Y., Yu S., Hong H.L., et al. Risk factors for isolation of multi-drug resistant organisms in coronavirus disease 2019 pneumonia: a multicenter study. Am J Infect Control. 2021;49:1256–1261. doi: 10.1016/j.ajic.2021.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seaton R.A., Cooper L., Gibbons C.L., Malcolm W., Choo-kang B., Griffith D., et al. Antibiotic prescribing for respiratory tract infection in patients with suspected and proven COVID-19: results from an antibiotic point prevalence survey in Scottish hospitals. JAC Antimicrob Resist. 2021;3:dlab078. doi: 10.1093/jacamr/dlab078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans T., Davidson H.C., Low J.M., Basarab M., Arnold A. Antibiotic usage and stewardship in patients with COVID-19: too much antibiotic in uncharted waters? J Infect Prevent. 2021;22:119–125. doi: 10.1177/1757177420976813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Public Health England . January 10, 2020. COVID-19: guidance for health professionals. Available at: www.gov.uk/government/collections/wuhan-novel-coronavirus [last accessed December 2021] [Google Scholar]
- 23.Stangerup M., Hansen M.B., Hansen R., Kostadinov K., Olesen B.S., Calum H. Hand hygiene compliance of healthcare workers before and during the COVID-19 pandemic: a long-term follow-up study. Am J Infect Control. 2021;49:1118–1122. doi: 10.1016/j.ajic.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]