Figure 1. Flow of Participants in the COVID-19 Antiplatelet Domain of the REMAP-CAP Randomized Clinical Trial.
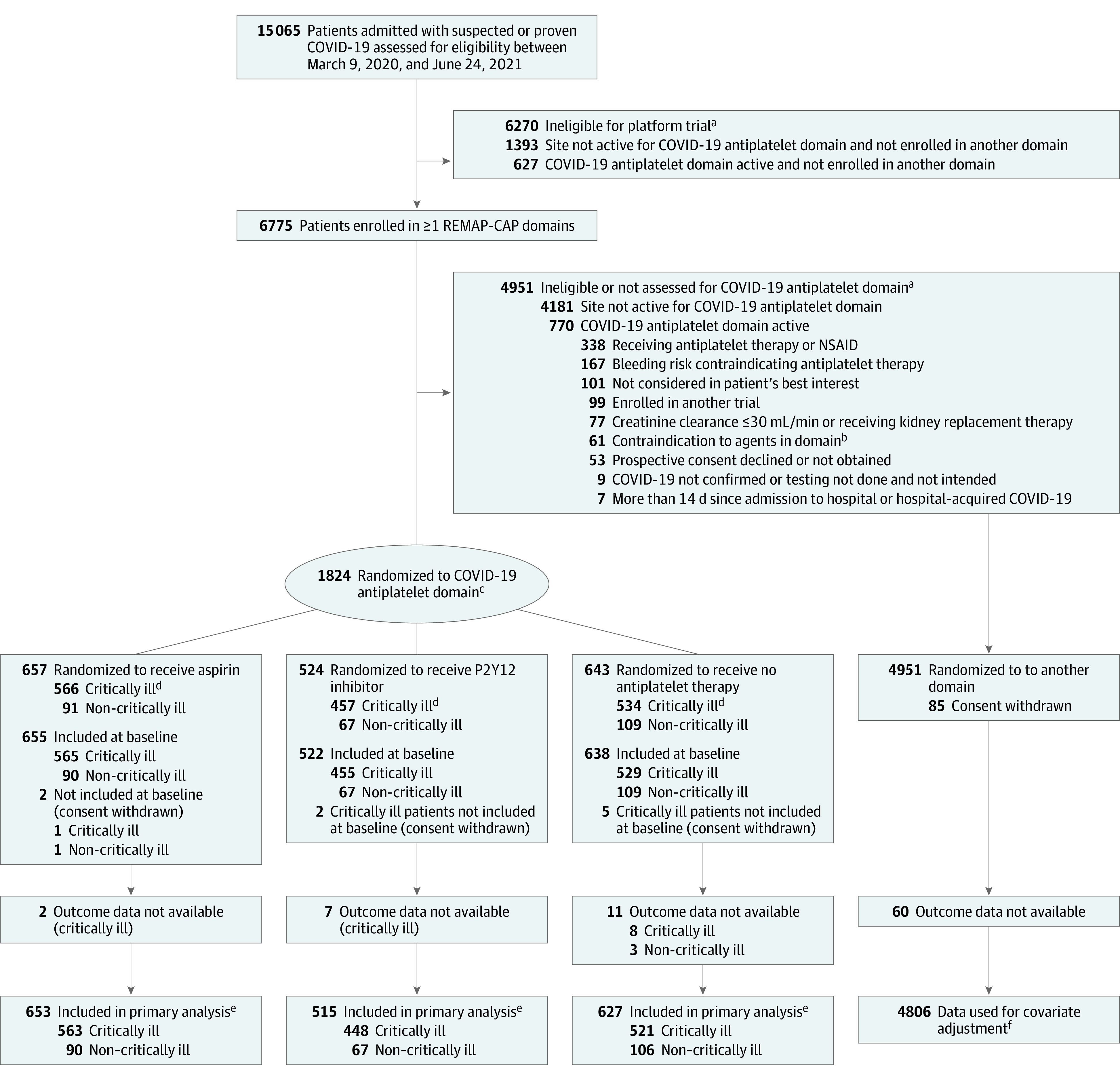
NSAID indicates nonsteroidal anti-inflammatory drug; REMAP-CAP, Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia. As a platform trial with a single master protocol (Supplement 1) and multiple treatments evaluated simultaneously, this trial applied eligibility criteria at the platform level and at the domain level. Patients had to be eligible for both platform and domain to be randomized. With an adaptive platform design, it allowed treatments to be stopped for futility, to declare 1 or more treatments to be superior, or to add new treatments or whole therapeutic areas during the course of the trial. A domain refers to a common therapeutic area (eg, antiviral therapy or immunoglobulin therapy) within which several interventions or intervention dosing strategies could be randomly assigned.
aPatients could meet more than 1 ineligibility criterion.
bContraindications to antiplatelet agents, including hypersensitivity to an antiplatelet agent specified as an intervention, excluded patients from receiving that antiplatelet intervention; known or suspected pregnancy excluded patients from the P2Y12 inhibitor intervention; administration or intention to administer lopinavir/ritonavir excluded patients from receiving a P2Y12 inhibitor at sites that were using clopidogrel and ticagrelor.
cParticipants were randomized via a centralized computer program to each intervention starting with balanced assignment and then adapted with preferential assignment to interventions that appeared most favorable until predefined statistical triggers of superiority or futility were met.
dCritically ill patients were required to have at least 1 of high-flow nasal cannula oxygenation, invasive or noninvasive mechanical ventilation, or vasopressor or inotropic infusion.
eResults for non–critically ill patients were used for borrowing within the primary model, meaning that results for non–critically ill patients were partially pooled with critically ill patients in the primary analysis. This partial pooling provides a more precise estimate of the treatment effect of antiplatelet therapy in critically ill patients if the observed data in the 2 groups are similar.
fThe primary analysis of alternative interventions within the antiplatelet domain is estimated from a model that adjusts for patient factors and for assignment to other interventions; all patients enrolled in the COVID-19 cohort for whom consent was obtained and follow-up data were available are included. The final estimate of an antiplatelet domain intervention’s effectiveness relative to any other within that domain is generated from patients who might have been randomized to either.
