Fig. 4. Kinetics of bromination as a function of the substrate concentrations for CeO2-x nanorods R=1 (A, B), R=4.5 (C, D) and R=9 (E, F).
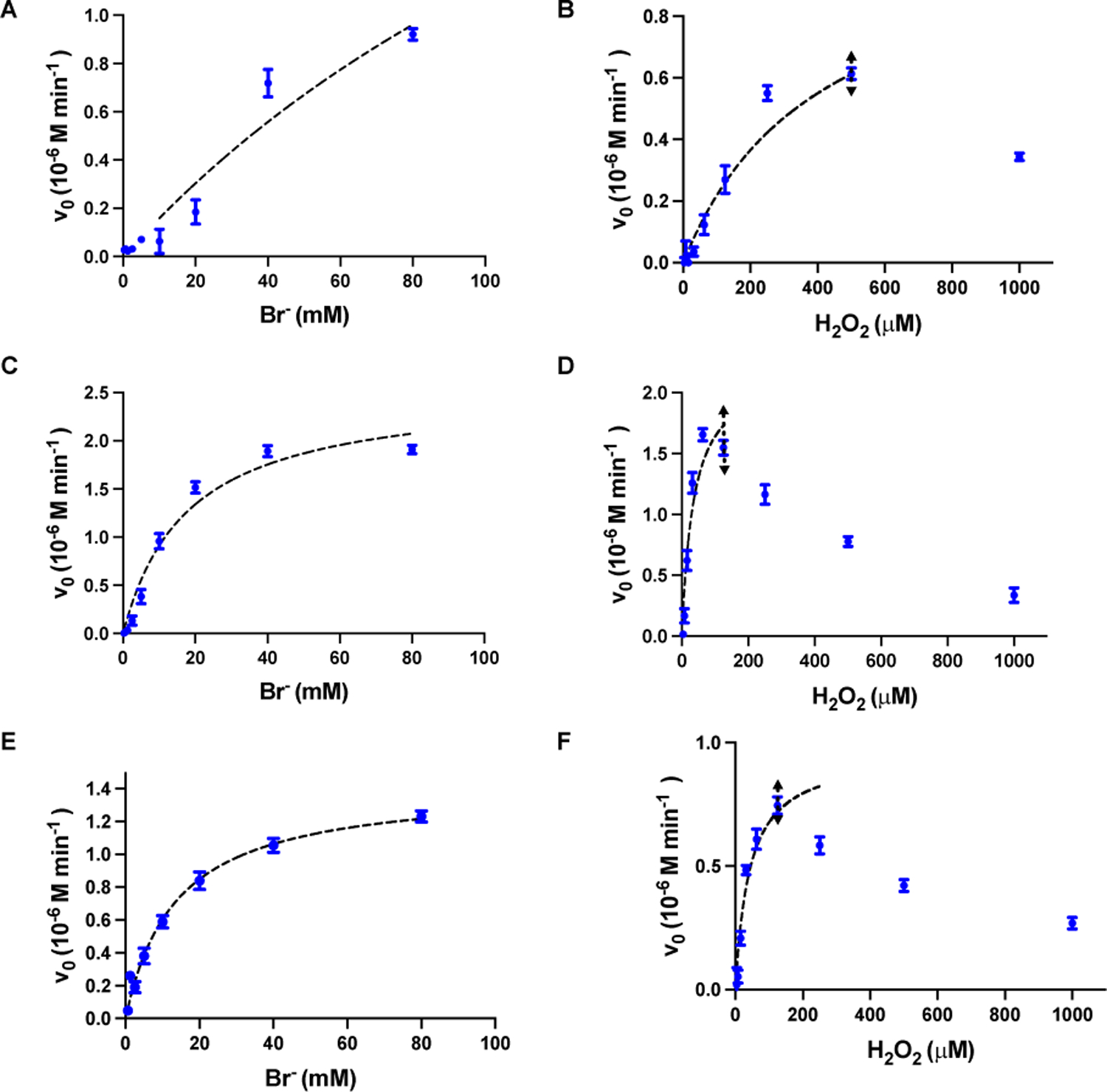
The values (blue dots) were fitted to the Michaelis-Menten equation (black dashed line). The concentration of CeO2-x nanorods was 0.04 mg/mL (in 5 mM MES buffer with 25 μM Phenol red). To obtain the Michaelis-Menten constant (Km) for Br−, the concentration of H2O2 were kept constant at 250 μM. To obtain the Km for H2O2, the concentration of Br− were kept constant at 10 mM. The fitting range for H2O2 is illustrated by the black double arrows.
