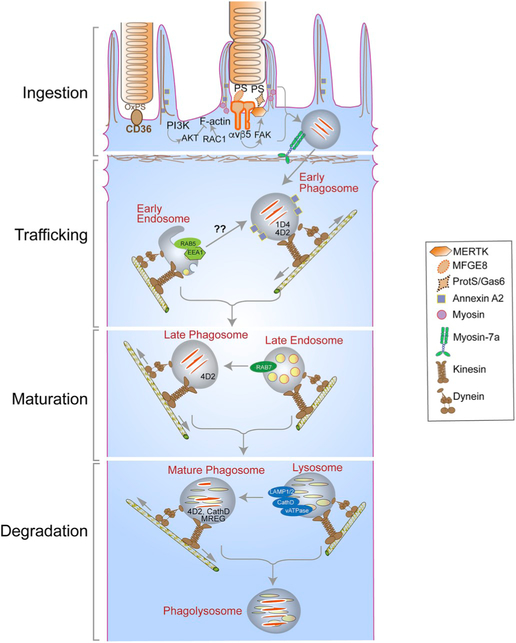
Fig. 7.
Major players and routes in the ingestion, degradation, and motility of photoreceptor outer segment (OS) phagosomes - Photoreceptor OS ingestion by the retinal pigment epithelium (RPE) occurs first, through the binding of exposed phosphatidylserine (PS) residues at the OS tips to the αvβ5 integrin on the apical RPE membrane, through its ligand milk fat globule protein E8 (MFGE8). OS recognition is then conveyed via focal adhesion kinase (FAK) to the ingestion receptor, Mer tyrosine kinase (MERTK), on the apical membrane, which also binds PS through its ligands, growth arrest-specific protein 6 (Gas6) or Protein S. CD36 binding to oxidized PS also serves as an additional ingestion pathway. OS binding results in actin remodeling to form the phagocytic cup that envelops and ingests the nascent phagosome, with the aid of a myosin motor. This remodeling is facilitated by the RAC1 GTPase and regulated by annexin A2 and, PI3K and AKT kinases. The ingested phagosome is first trafficked on apical actin filaments by myosin-7a, before being transferred to microtubule motors, kinesin and dynein, which mediate bidirectional motility with an overall apical to basal migration of phagosomes. These movements also facilitate phagosome interaction with early endosomes (indicated by RAB5 or early endosome antigen 1 [EEA1]), late endosomes (indicated by RAB7), and finally lysosomes (indicated by lysosome-associated membrane protein [LAMP] 1/2). During these interactions, the phagosome is gradually acidified through the vacuolar H+-ATPase (v-ATPase) and acquires degradative enzymes such as cathepsin D (CathD) which, in turn, requires melanoregulin (MREG) for enzyme maturation. The C-terminal epitope of rhodopsin, recognized by the mAb1D4 antibody, is lost early in phagosome maturation, whereas the N-terminus of rhodopsin remains mostly intact and recognizable by the mAb4D2 antibody.