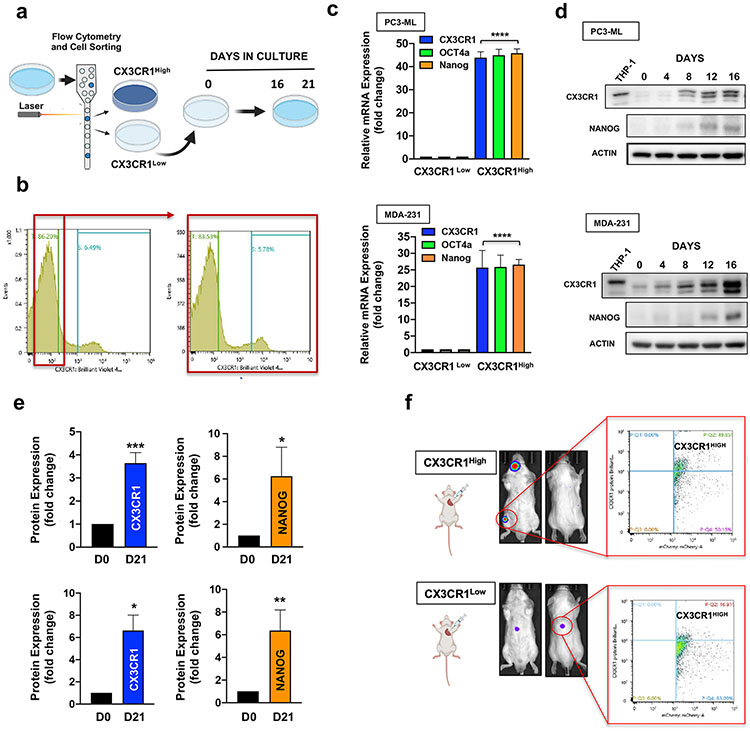
Figure 6.
CX3CR1Low cells exhibit phenotypic plasticity. a. CX3CR1Low cells were sorted from MDA-231 and PC3-ML parental cell lines and placed in culture for 16 days (western blotting) or 21 days (flow cytometry, FACS, transcript analysis and western blotting). b. After 21 days the cultures that started as CX3CR1Low pure cells showed the re-emergence of CX3CR1High phenotypes, assessed by flow cytometry. The newly generated CX3CR1High cells were isolated by FACS and, when the transcript levels of CX3CR1, OCT4a, and NANOG were quantified, they showed significantly higher levels of all three transcripts as compared to the CX3CR1Low cells (c). d. CX3CR1Low cells were isolated from MDA-231 and PC3-ML parental cells and placed in culture for 16 days and cell lysates collected every four days. Western blotting analysis showed that in these conditions CX3CR1 re-expression occurred between 4 and 8 days of culture, while NANOG protein started being re-expressed between 8 and 12 days of culture. THP-1, a human monocytic cell line, served as a positive control for CX3CR1 protein detection. e. The increase in CX3CR1 and NANOG protein levels was assessed by densitometry and compared between CX3CR1Low cells immediately after sorting and after 21 days in culture. f. The tumors generated from intracardiac inoculation of CX3CR1Low and CX3CR1High cells sorted from PC3-ML parental line were dissociated for flow cytometry analysis of CX3CR1 cell-surface expression. The single skeletal tumor generated by pure CX3CR1Low cells harbored 17% of CX3CR1High cells, indicating the occurrence of phenotypic plasticity in vivo. The skeletal tumors generated by pure CX3CR1High cells contained higher fractions of CX3CR1High phenotypes (35±4%) as compared to the parental cell line cultured in vitro (see Fig. 3). (c: ****P<0.0001 (MDA-231); ****P<0.0001 (PC3-ML); e: ***P=0.0005; *P=0.03 (Top: PC3-ML); *P=0.01;**P=0.007 (Bottom: MDA-231). One-way ANOVA or Student’s t-test).