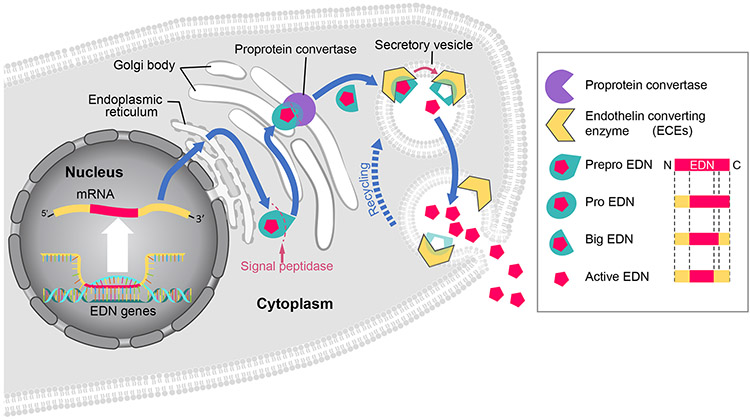
Figure 1. Synthesis and release of endothelin (EDN).
EDN mRNA is translated into the preproEDN form located in the endoplasmic reticulum, where the N-terminal signal sequence is cleaved by a signal peptidase into proEDN. The proEDN is transferred to Golgi body and cleaved into bigEDN by a proprotein convertase. The bigEDN is then encapsulated by secretory vesicles where it is cleaved into a bioactive form by endothelin converting enzyme (ECE) that is located on the membranes of secretory vesicles. As secretory vesicles fuse with the plasma membrane, EDN is released to the extracellular space. Although each EDN isoform is translated into early proteins of different sizes, all three follow the same synthesis process and become 21-amino acid peptides in their bioactive form.