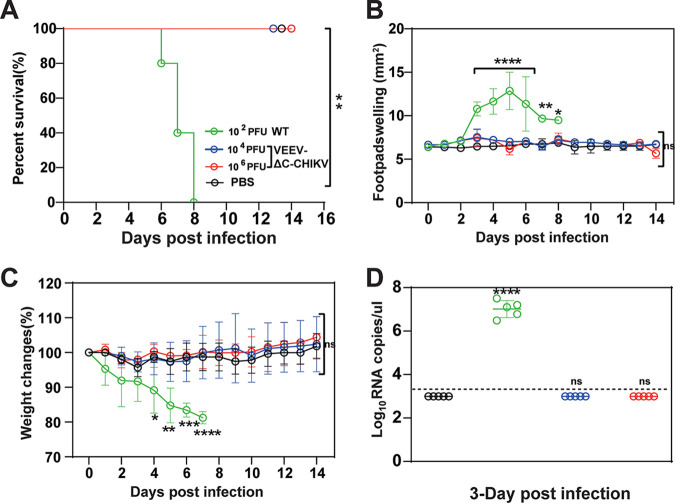
FIG 8.
Safety evaluation of VEEV-ΔC-CHIKV in the IFNAR−/− mouse model. Four groups of 6-weeks-old IFNAR−/− mice (n = 5 per group) were infected s.c. in the ventral/lateral side of the hind foot with different doses of WT CHIKV or VEEV-ΔC-CHIKV. The PBS infection was used as mock immunization. Animal survival (A), footpad swelling (B), and weight loss (C) as well as viremia (D) were monitored daily until 14 days postinoculation. Student's t test or two-way ANOVA was used to determine statistical differences between groups. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, respectively.