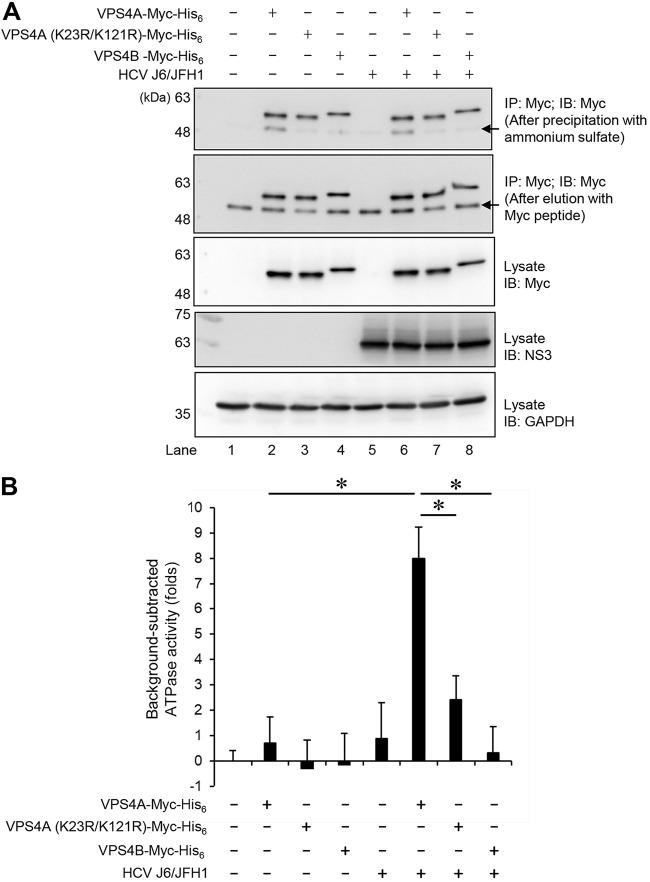
FIG 10.
HCV infection enhances the ATPase activity of VPS4A via VPS4A polyubiquitylation. Huh-7.5 cells were infected with HCV J6/JFH1 at an MOI of 2. At 3 h postinfection, HCV-infected cells or mock-infected control cells were transfected with pEF1A-VPS4A-Myc-His6, pEF1A-VPS4A (K23R/K121R)-Myc-His6, or pEF1A-VPS4B-Myc-His6 as indicated. At 4 days after transfection, cells were harvested, and cell lysates were immunoprecipitated with anti-Myc beads, followed by elution with Myc peptide. The eluate was precipitated with 3.2 M ammonium sulfate, followed by resuspending in ATPase assay buffer. (A) The purified proteins were subjected to immunoblotting with anti-Myc MAb (1st panel, after precipitation with ammonium sulfate; 2nd panel, after elution with Myc peptide). Input samples were immunoblotted with anti-Myc MAb (3rd panel), anti-NS3 MAb (4th panel), and anti-GAPDH MAb (5th panel), respectively. The level of GAPDH served as a loading control. Arrows indicate immunoglobulin heavy chain. (B) ATPase activities of VPS4A-Myc-His6, VPS4A K23R/K121R-Myc-His6, or VPS4B-Myc-His6 expressed in HCV-infected cells or mock-infected controls cells were measured by ATPase assay. The value of mock-infected controls cells was used as background and was arbitrarily defined as 0. Background-subtracted ATPase activities were shown. Data represent means ± SEM of data from three independent experiments, *, P < 0.05.