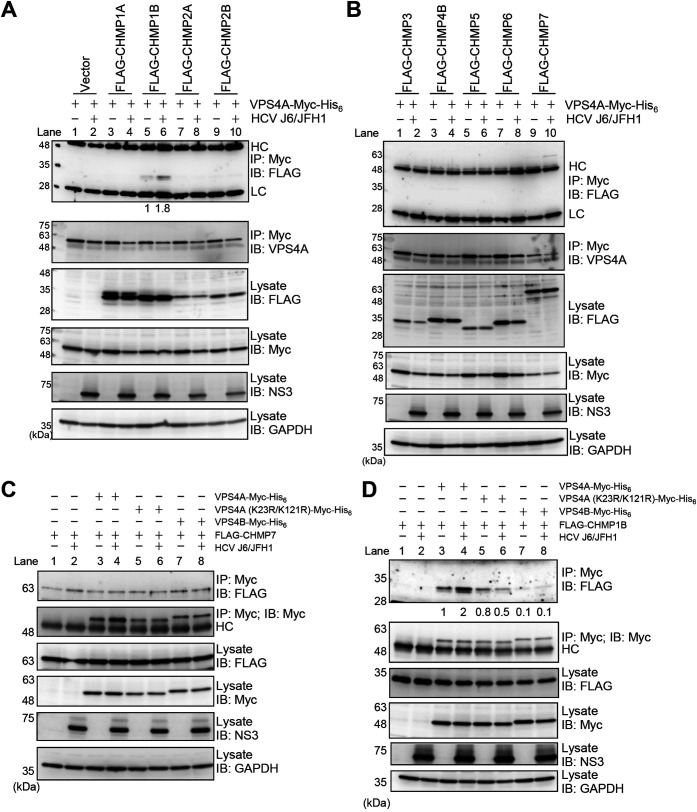
FIG 9.
HCV infection enhances the interaction between VPS4A and CHMP1B via VPS4A polyubiquitylation. (A and B) Huh-7.5 cells were infected with HCV J6/JFH1 at an MOI of 2. At 3 h postinfection, cells were cotransfected with pEF1A-VPS4A-myc-His6 and the indicated 3×FLAG-tagged CHMP plasmids. At 4 days transfection, cells were harvested, and cell lysates were immunoprecipitated with anti-Myc beads, followed by immunoblotting with anti-FLAG PAb (1st panel) or anti-VPS4A PAb (2nd panel). The relative levels of coimmunoprecipitated FLAG-tagged CHMP1B protein were quantitated by densitometry and indicated below in the respective lanes. Input samples were immunoblotted with anti-FLAG PAb (3rd panel), anti-Myc MAb (4th panel), anti-NS3 MAb (5th panel), and anti-GAPDH MAb (6th panel), respectively. The level of GAPDH served as a loading control. HC, immunoglobulin heavy chain; LC, immunoglobulin light chain. Huh-7.5 cells were infected with HCV J6/JFH1 at an MOI of 2. At 3 h postinfection, cells were cotransfected with pCMV-3×FLAG-CHMP7 (C) or pCMV-3×FLAG-CHMP1B (D) and pEF1A-VPS4A-Myc-His6, or pEF1A-VPS4A (K23R/K121R)-Myc-His6, or pEF1A-VPS4B-Myc-His6 as indicated. At 4 days transfection, cells were harvested, and cell lysates were immunoprecipitated with anti-Myc beads, followed by immunoblotting with anti-FLAG PAb (1st panel) or anti-Myc MAb (2nd panel). The relative levels of coimmunoprecipitated FLAG-tagged CHMP1B protein were quantitated by densitometry and indicated below in the respective lanes. Input samples were immunoblotted with anti-FLAG PAb (3rd panel), anti-Myc MAb (4th panel), anti-NS3 MAb (5th panel), anti-GAPDH MAb (6th panel), respectively. The level of GAPDH served as a loading control. HC, immunoglobulin heavy chain.