Abstract
Objective
Registers of diagnoses and treatments exist in different forms in the European countries and are potential sources to answer important research questions. Prevalence and incidence of thyroid diseases are highly dependent on iodine intake and, thus, iodine deficiency disease prevention programs. We aimed to collect European register data on thyroid outcomes to compare the rates between countries/regions with different iodine status and prevention programs.
Design
Register-based cross-sectional study.
Methods
National register data on thyroid diagnoses and treatments were requested from 23 European countries/regions. The provided data were critically assessed for suitability for comparison between countries/regions. Sex- and age-standardized rates were calculated.
Results
Register data on ≥1 thyroid diagnoses or treatments were available from 22 countries/regions. After critical assessment, data on medication, surgery, and cancer were found suitable for comparison between 9, 10, and 13 countries/regions, respectively. Higher rates of antithyroid medication and thyroid surgery for benign disease and lower rates of thyroid hormone therapy were found for countries with iodine insufficiency before approx. 2001, and no relationship was observed with recent iodine intake or prevention programs.
Conclusions
The collation of register data on thyroid outcomes from European countries is impeded by a high degree of heterogeneity in the availability and quality of data between countries. Nevertheless, a relationship between historic iodine intake and rates of treatments for hyper- and hypothyroid disorders is indicated. This study illustrates both the challenges and the potential for the application of register data of thyroid outcomes across Europe.
Keywords: Europe, registries, iodine, thyroid diseases
Introduction
Register-based data on treatments and diagnoses of thyroid disease can be used as proxies for thyroid disease frequency in a population. This has, for example, been successfully utilized in Denmark (1). In many European countries, treatments for thyroid disease and/or diagnoses of thyroid disorders are documented in nationwide or regional registers. This encompasses registers of diseases, medications and other treatments, hospital admissions, and ambulatory care, as well as registries related to health insurance. Ideally, for use in research projects, a register should cover the entire country/region or a large, well-defined sub-population whereby it is representative, and the data should be of acceptable validity (2).
European registers have previously been collated for research projects in, for example, melanoma (3), asthma (4), and cerebral palsy (5) and several initiatives to promote and improve the collaboration and utilization of registers in Europe are established (6, 7). However, the availability and applicability of register data for assessment of thyroid outcomes from the European countries have not yet been examined. After a thorough assessment of the quality and comparability of data across countries, European registers can potentially represent valuable and efficient data sources for evaluating differences in thyroid outcomes across the countries/regions.
Iodine intake in a population is a main factor behind thyroid disease epidemiology. Iodine deficiency is associated with adverse outcomes ranging from severe mental retardation and stunting to goiter/thyroid nodules and hyperthyroidism (8). However, the window of adequate iodine intake is narrow, hence, iodine excess may increase the risk of hypothyroidism and autoimmune thyroid disorders in a population (9).
Most regions of Europe will be iodine deficient without programs to prevent iodine deficiency disorders (IDD). However, the implementation and type of IDD prevention programs depend on stakeholder barriers, political prioritization (10), and cultural differences. The IDD prevention programs range from general recommendations for intake of iodine-rich foods, over recommendations for iodine supplementation especially for pregnant and lactating women, to voluntary or mandatory iodine fortification (IF), most commonly addition of iodine to table salt and salt in bread (11). An overview of IF programs and iodine intake across 23 European countries/regions are presented in Table 1. The optimal level of iodine intake and the most effective IDD prevention program remains to be established.
Table 1.
IDD prevention and iodine intake in the European countries.
| Country | Country code | Iodine fortification strategy (13, 14, 15, 16, 17, 18) | Iodine intake, Median UIC, μg/L (population, year) (18, 19, 20, 21, 22, 23) |
|---|---|---|---|
| Belgium | BE | Voluntary fortification of salt, 10–15 ppm since 2009 | Insufficient, 80 (SAC, 1998) Adequate, 113 (SAC, 2010–2011) |
| Bulgaria | BG | Mandatory fortification of salt, 28–55 ppm since 1994/1996 | Adequate, 111 (SAC, 1996) Adequate, 182 (SAC, 2008) Adequate, 176 (SAC, 2012) |
| Croatia | HR | Mandatory fortification of salt, 19 ppm since 2011 | Adequate, 140 (SAC, 2002) Adequate, 248 (SAC, 2009) |
| Czech Republic | CZ | Voluntary fortification of salt, 27 ppm since 2016 | Adequate, 119 (SAC, 2000) Adequate 163 (SAC, 2010) |
| Denmark | DK | Mandatory fortification of salt, 13 ppm since 2000 | Insufficient, 61 (A, 1997–1998) Adequate, 101 (A, 2004–2005) Adequate, 145 (SAC, 2015) |
| Finland | FI | Voluntary fortification of salt, 25 ppm since approx. 1946 | Adequate, 164 (A, 1997) Insufficient, 83 (A, 2013) |
| Germany | DE | Voluntary fortification of salt, 20 ppm since 1993 | Adequate, 148 (SAC, 1999) Adequate, 122 (SAC, 2003–2006) Insufficient, 89 (SAC+A, 2014–2017) |
| United Kingdom | UK | ||
| Northern Ireland | None | Adequate, 111 (SAC, 2014–2015) | |
| Scotland | None | N/A | |
| Greece | GR | Voluntary fortification of salt, 30 ppm since 1987 | Adequate, 202 (2001) Adequate, 132 (A, 2017–2018) |
| Hungary | HU | Mandatory fortification of salt, 19 ppm since 2013 | Insufficient, 80 (SAC, 1994–1997) Adequate, 228 (SAC, 2005) |
| Iceland | IS | None | Adequate, 150 (A, 1998) Adequate, 200 (A, 2007–2008) |
| Ireland | IE | None | Insufficient, 82 (A, 1999) Adequate, 111 (SAC, 2014–2015) |
| Israel | IL | None | Insufficient, 83 (SAC, 2016) |
| Latvia | LV | Voluntary fortification of salt, 20–50 (40–100) ppm since 2005 | Insufficient, 59 (SAC, 2000) Adequate, 110 (SAC, 2010–2011) |
| North Macedonia | MK | Mandatory fortification of salt, 25 ppm since 1999 | Adequate, 241 (2007) Adequate, 216 (2016) |
| Norway | NO | Voluntary fortification of salt, 5 ppm since 1938 | Adequate, 104 (1999–2001) Insufficient, 75 (A, 2017–2018) |
| Poland | PL | Mandatory fortification of salt, 23 ppm since 1997 | Insufficient, 84 (SAC, 1999) Adequate, 112 (SAC, 2009–2011) Adequate, 120 (SAC, 2017) |
| Portugal | PT | None | Adequate, 106 (SAC, 2010) |
| Slovenia | SI | Mandatory fortification of salt, 19 ppm since 1999 | Adequate, 140 (A, 2003–2005) |
| Spain | ES | Voluntary fortification of salt, 60 ppm since 1983 | Adequate, 109 (SAC, 1995, 2000–2002) Adequate, 117 (2009–2010) Adequate, 173 (SAC, 2011–2012) |
| Sweden | SE | Voluntary fortification of salt, 10 ppm since 1936, 50 ppm since 1966 | Adequate, 125 (SAC, 2006–2007) |
| Switzerland | CH | Voluntary fortification of salt, 25 ppm since 2014 | Adequate, 115 (SAC, 1999) Adequate, 120 (2009) Adequate, 137 (SAC, 2015) |
A, adults; N/A, not available; SAC, school-aged children; UIC, urinary iodine concentration.
In many countries, the assessment of median urinary iodine concentration in representative population samples is used to classify the iodine status and to steer IDD prevention programs. While this approach is relatively simple, it is not sufficient to evaluate the aim of such prevention programs, which is to reduce the prevalence and incidence of thyroid diseases and related treatments and to save expenditures in healthcare systems (12). This can potentially be achieved by analyses of register data. We hypothesized that efficient IDD prevention programs ensuring sufficient iodine intake are robustly associated with a low burden of thyroid-related diseases in populations.
The EUthyroid project was an ambitious collaborative project with participation and representatives from most EU countries funded by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 634453. The overall aim of the EUthyroid project was to ‘evaluate iodine deficiency prevention and monitoring programs in European countries, to initiate capacity building for harmonized European ID prevention and monitoring programs’.
In this paper, we report lessons and results from work package 1 of the EUthyroid project. Firstly, we evaluate the availability, applicability, and comparability of the register data on thyroid outcomes in the European countries, and second, we report country-specific rates of thyroid outcomes and seek to identify patterns in the relationship with iodine supply across Europe.
Materials and methods
Register data
Representatives from each country participating in the EUthyroid project were requested to deliver national register data on diagnoses, medical treatments, and procedures for thyroid disorders. All data were requested for the calendar years 2011–2014 and stratified by sex and age in 5-year intervals. If this was not available, we allowed for the available data that best approximated these criteria, for example, data from 2015, data from a well-defined sub-population, or data in 10-year age intervals. Specific diagnoses and treatments requested were:
Diagnoses: Annual number of persons diagnosed with thyroid cancer (ICD-10: C73), congenital iodine-deficiency syndrome (ICD-10: E00), iodine-deficiency-related thyroid disorders and allied conditions (ICD-10: E01), subclinical iodine-deficiency (ICD-10: E02), other hypothyroidism (ICD-10: E03), other nontoxic goiter (ICD-10: E04), thyrotoxicosis/hyperthyroidism (ICD-10: E05), thyroiditis (ICD-10: E06), other thyroid disorders (ICD-10: E07), and one or more thyroid related diagnoses (ICD-10: E00-E07).
Treatments: Annual number of persons with at least one dispensed dose and total number of defined daily doses of antithyroid medication and thyroid hormone therapy. Annual number of persons with at least one procedure and the total number procedures for all thyroid surgeries, thyroid surgeries with benign indication, all radioiodine treatments, radioiodine treatments with benign indication, and fine needle biopsies.
The demographic data of the total populations (total number of individuals and stratified by sex and 5-year age intervals) were derived from Eurostat (http://ec.europa.eu/eurostat) or provided from the national EUthyroid representatives for regional or sub-national populations.
Ethical and/or data handling approvals were obtained in each country according to national regulations to obtain aggregated register data. Only aggregated data were collected and, thus, no patient-level data (personal data) were handled thereby protecting the privacy of the individuals.
Critical assessment and selection of data and variables
The national representatives were also requested to provide a description of the data sources and health care organization factors relevant to assess the completeness and validity of the data provided.
The applicability of the provided data from each country was critically assessed. For inclusion in the database and calculation of raw rates the provided data had to fulfill the criteria for the requested data. For comparison between countries and calculation of standardized rates, the data and variables had to be stratified by sex and age, collected from comparable data sources, and registered by comparable methods. The critical assessment was conducted by the senior author, BHT.
Iodine intake
Populations’ iodine intakes were derived from published reports of urinary iodine concentrations in school-aged children or adults and summarized in Table 1. Iodine intake was classified as insufficient, adequate, or excess according to current WHO epidemiological recommendations (24). Early and recent iodine intake were classified as iodine intake approximately 15–20 years before and after 2001, respectively.
Statistical methods
Rates of medications were calculated as 1-year period prevalence per 10,000 persons. Rates of diagnoses were calculated per year per 10,000 persons. Raw rates were calculated with the treatments and diagnoses as the numerator and the demographic data of the population as the denominator in the provided sex and/or age strata where possible.
Standardized rates were calculated to remove the effect of the differences in sex and age distributions between the European countries. This was done by calculating the proportion of each sex and age-specific strata in the total European Standard Population (25) and multiplying this by the raw rate in the corresponding strata for each country before summarizing the overall standardized rate in each country. When data were provided for more than one calendar year, the rates were calculated as the average of the calendar years available.
Comparisons between countries were done using standardized rates. No formal statistical tests were applied in the comparison of rates between countries.
Results
Data availability and data assessment
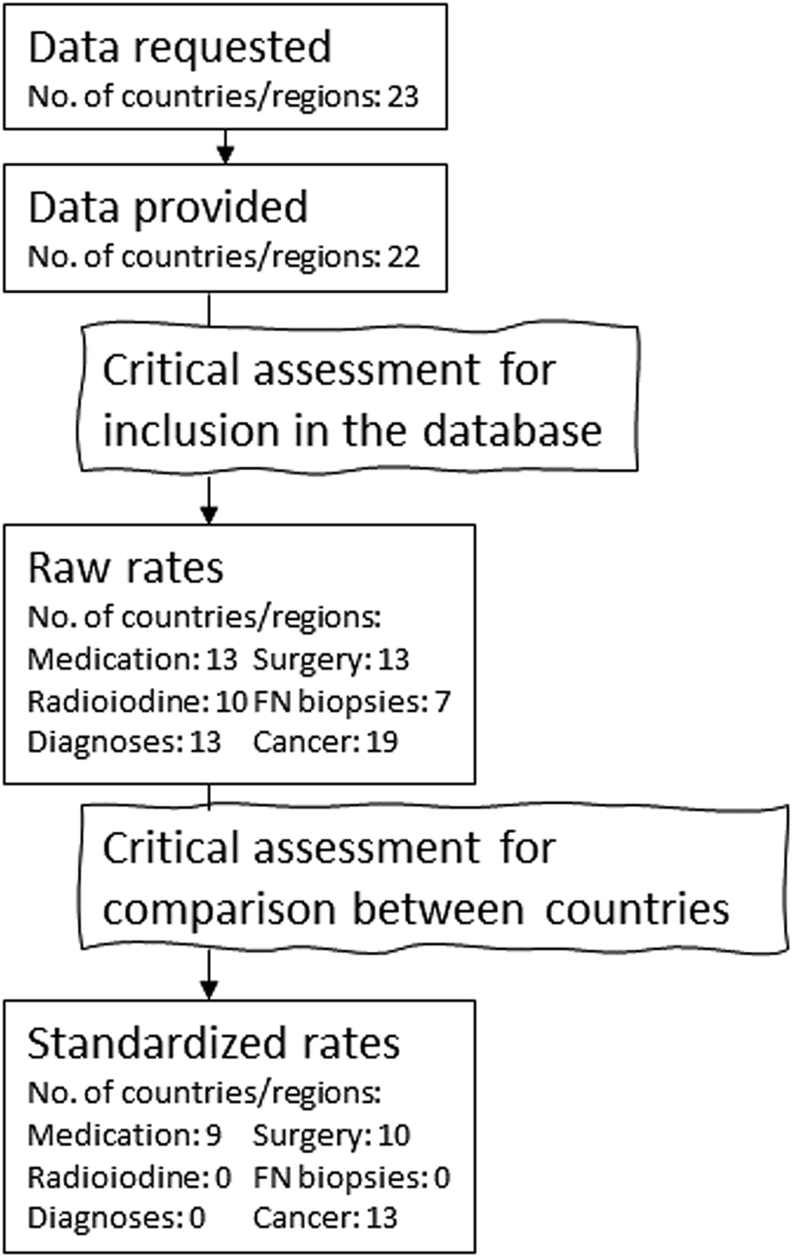
Table 2 provides an overview of the data sources and Fig. 1 provides an overview of the flow of the data acquisition, critical assessments, and calculations.
Table 2.
Data requested and provided with stratification level and area.
| Country | Country code | Thyroid medication | Thyroid surgery | Radio-iodine | Fine needle biopsies | Diagnoses of thyroid disease | Diagnoses of thyroid cancer |
|---|---|---|---|---|---|---|---|
| Belgium | BE | Sex/age | Sex/age | Sex/age | Sex/age | N/A | Sex/age |
| Bulgaria | BG | Sex | Sex | Total | Sex | Sex | Sex/age |
| Croatia | HR | N/A | N/A | N/A | N/A | N/A | Sex |
| Czech Republic | CZ | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex |
| Denmark | DK | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Finland | FI | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Germany | DE | Sex/age a | N/A | N/A | N/A | Sex/agea | N/A |
| United Kingdom | UK | ||||||
| Northern Ireland | N/A | N/A | N/A | N/A | N/A | Sex/age | |
| Scotland | N/A | N/A | N/A | N/A | N/A | Sex/age | |
| Greece | GR | Totala | N/A | Totala | N/A | Totala | Totala |
| Hungary | HU | Sex/age | Sex/age | Sex/age | N/A | Age | Age |
| Iceland | IS | Sex | N/A | N/A | N/A | N/A | Sex |
| Ireland | IE | N/A | N/A | N/A | N/A | N/A | Sex/age |
| Israel | IL | Sex/age | N/A | N/A | N/A | Sex/age | Sex/age |
| Latvia | LV | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| North Macedonia | MK | Totala | N/A | N/A | N/A | N/A | N/A |
| Norway | NO | Sex/age | Sex/age | N/A | N/A | Sex/age | Sex/age |
| Poland | PL | Totala | Total | N/A | N/A | Total | Sex/age |
| Portugal | PT | Totala | Sex/age | Sex/agea | N/A | Sex/age | Sex/Age |
| Slovenia | SI | Total | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Spain | ES | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Sweden | SE | Sex/age | Sex/age | Total | N/A | Sex/age | Sex/age |
| Switzerland | CH | N/A | N/A | N/A | N/A | N/A | N/A |
Bold indicates National data; Italics indicate regional data or data from less defined sub-populations.
aData not suitable for inclusion in the database and calculation of raw rates.
N/A, not available.
Figure 1.
Overview of data availability, critical assessments, and calculation of rates.
Register data for at least one thyroid outcome was available and provided from 22 out of 23 participating countries/regions. However, the critical assessment concluded that data from three countries were considered unsuitable to be included in the database. See an overview of data and assessments in the Supplementary Tables 1 and 2 (see section on supplementary materials given at the end of this article). Raw rates for data included in the database are presented in the Supplementary Tables 3, 4, and 5.
The outcome variables were grouped into six categories: thyroid medication, thyroid surgery, radioiodine treatment, fine needle biopsies, diagnoses of thyroid diseases, and diagnoses of incident thyroid cancer. Sex- and age-stratified data from all categories were available from four countries, whereas data from only one outcome variable were available from four countries. The critical assessment of the data included in the database found that data on the use of thyroid medication, number of thyroid surgeries, and incidence of thyroid cancer were of acceptable quality and thus appropriate for calculation of standardized rates and comparison between countries.
In contrast, data on diagnoses of thyroid diseases except for thyroid cancer were not considered suitable for comparisons across countries. In some countries, registrations included patients treated in primary care whereas only patients attending hospitals were included in other countries, and it was not possible to distinguish between incidence and prevalence data. Furthermore, in some countries registration of diagnoses was based on the ICD-9 system whereas the ICD-10 system was used in other countries and the conversion of data from ICD-9 to ICD-10 caused some misclassification. Data on radioiodine and fine-needle biopsies were only available from a few countries and were generally considered unreliable.
Thyroid medication
Data from nine countries were found to be appropriate for comparison of standardized rates of thyroid medication between countries. Hereby we found that the use of antithyroid medication was lowest for Sweden, Finland, Norway, and Spain (8.6–13.7 users/10,000 persons) and highest for Denmark, Latvia, and Hungary (34.8–43.2 users/10,000 persons). Conversely, the use of thyroid hormone therapy was lowest for Latvia, Denmark, and Hungary (192.4–253.1 users/10,000 persons) and highest for the Czech Republic, Belgium, Finland, Sweden, and Norway (424.8–612.8 users/10,000 persons) (Table 3).
Table 3.
Thyroid medication use per 10,000 persons, sex- and age-standardized.
| Country | Country code | Thyroid medication | |||
|---|---|---|---|---|---|
| Antithyroid medication, users | Antithyroid medication, DDD | Thyroid hormone therapy, users | Thyroid hormone therapy, DDD | ||
| Belgium | BE | 19.28 | 481.46 | ||
| Czech Republic | CZ | 26.60 | 612.83 | ||
| Denmark | DK | 43.19 | 8825 | 241.76 | 58,356 |
| Finland | FI | 9.94 | 513.95 | ||
| Hungary | HU | 34.78 | 4386 | 253.09 | 39,040 |
| Latvia | LV | 41.51 | 5372 | 192.36 | 30,901 |
| Norway | NO | 13.12 | 424.78 | ||
| Spain | ES | 13.71 | 1672 | 308.24 | 55,989 |
| Sweden | SE | 8.59 | 3235 | 432.84 | 91,269 |
DDD, defined daily dose.
There was a clear indication of higher use of antithyroid medication relative to thyroid hormone therapy for countries with early iodine insufficiency. However, there was no apparent pattern in the association between recent iodine intake and use of thyroid medications.
Thyroid surgeries
For thyroid surgeries, data from 10 countries were found to be appropriate for comparison between countries. We found that the standardized rates of thyroid surgeries for benign indications were lowest for the Czech Republic, Slovenia, Norway, Sweden, and Spain (1.4–2.0 patients/10,000 persons), all with early iodine adequacy, and highest for Belgium, Latvia, and Hungary (4.3–5.1 patients/10,000 persons), all with early iodine insufficiency (Table 4). However, no clear pattern was indicated for thyroid surgeries for all indications or when compared with recent iodine intake.
Table 4.
Thyroid surgeries per 10,000 persons, sex- and age-standardized.
| Country | Country code | Thyroid surgery | |||
|---|---|---|---|---|---|
| All indications, number of persons | All indications, number of procedures | Benign indications, number of persons | Benign indications, number of procedures | ||
| Belgium | BE | 5.67 | 5.05 | ||
| Czech Republic | CZ | 5.94 | 6.11 | 1.42 | 1.55 |
| Denmark | DK | 3.77 | 4.10 | 3.37 | 3.51 |
| Finland | FI | 4.12 | 4.38 | 3.68 | 3.79 |
| Hungary | HU | 4.57 | 5.27 | 4.29 | 4.83 |
| Latvia | LV | 5.20 | 5.31 | 4.73 | 4.76 |
| Norway | NO | 3.20 | 3.28 | 1.99 | 2.00 |
| Slovenia | SI | 1.57 | 1.57 | ||
| Spain | ES | 2.77 | 2.27 | ||
| Sweden | SE | 5.35 | 5.53 | 2.20 | 2.22 |
Thyroid cancer
Data on standardized rates of thyroid cancer diagnoses were assessed to be appropriate for comparison between 13 countries/regions. The lowest rates were found in Bulgaria, Northern Ireland, and Scotland (0.4–0.5 patients/10,000 persons) and the highest rates in Denmark, Latvia, and Hungary (0.9–1.4 patients/10,000 persons) (Table 5), with no clear pattern in the association between early or recent iodine intake and cancer rates.
Table 5.
Incident thyroid cancer per 10,000 persons, sex- and age-standardized.
| Country | Country code | Diagnoses of thyroid cancer |
|---|---|---|
| Belgium | BE | 0.87 |
| Bulgaria | BG | 0.40 |
| Denmark | DK | 1.38 |
| Finland | FI | 0.90 |
| Great Britain | UK | |
| Northern Ireland | 0.46 | |
| Scotland | 0.49 | |
| Hungary | HU | 0.91 |
| Ireland | IE | 0.65 |
| Latvia | LV | 1.12 |
| Norway | NO | 0.73 |
| Slovenia | SI | 0.72 |
| Spain | ES | 0.89 |
| Sweden | SE | 0.62 |
Discussion
In this first collation of register data on thyroid outcomes from European countries, we found a high degree of heterogeneity in the availability and quality of register data between countries. Data for thyroid medication, thyroid surgery, and thyroid cancer were suitable for comparison between 9, 10, and 13 countries, respectively. These data indicated higher rates of antithyroid medication and thyroid surgery for benign disease and lower rates of thyroid hormone therapy in countries with early iodine insufficiency. There was no apparent pattern in the association between thyroid cancer and iodine intake.
Registers, data availability, and data quality
All but one of the 23 participating countries had data on at least one thyroid outcome. However, the data sources, the number of variables. and the quality of register data varied widely due to differences in the organization of the healthcare systems, digitization, history/culture, and political decisions. This is also recognized in previous papers acknowledging the vast potential of the European registers but also addressing the challenges (6, 7, 26).
Nationwide health registries were established in the Scandinavian countries, Latvia, Hungary, and in the Region of Catalonia in Spain. In contrast, Croatia, Germany, Greece, Great Britain, North Macedonia, and Switzerland provided no data from registers fulfilling the requested criteria. Register data from the Czech Republic, Belgium, and Bulgaria were derived from health insurance companies covering the majority of the population or a well-defined sub-population. These encompass both public and private health insurance companies. Information on thyroid cancer was available from most countries.
For all data sources, the representativity and validity were assessed. There was little concern regarding the representativity in the Nordic registers (27), but some health insurance registers may carry a risk of social stratification hampering the representativity. European cancer registries have a long history and the coverage is generally high (6). The validity of registers could be compromised by over-, under-, or misreporting. Thyroid medications require prescriptions in most European countries and are rarely prescribed in hospitals. This increases the probability of correct reporting as most registers are based on redeemed prescriptions (26). However, for example, in the Latvian National Health Service register, only reimbursed prescriptions were registered. Some patients may have private insurance or choose to pay fully out-of-pocket and are thus not registered. The validity of thyroid surgeries was also assessed to be appropriate for the included countries. In cancer registries, the data are generally reliable but comparability is still somewhat influenced by diverse methods between countries (6). In contrast, diagnoses other than cancer were considered to be unreliable. Registration of thyroid diagnoses varies both between countries and can also vary within countries between health care providers and specialists; for example, the rates of congenital iodine-deficiency syndrome were far higher in some countries than what national experts regard as plausible. Validation studies are rarely available for any of the registers (26).
In addition, between-country differences in other risk factors for thyroid outcomes (e.g. alcohol (28, 29), smoking (30), and other micronutrient deficiencies (31)) and between-country differences in diagnostic activity and treatment practices may also influence the rates of thyroid outcomes independently of iodine intake. Especially differences in ultrasound utilization can impact the detection and thus diagnoses and treatment for thyroid nodules and cancer. Despite clinical practice guidelines developed by recognized professional associations, surveys among clinicians find some variability and divergence from guidelines in the clinical management of Grave’s disease, hypothyroidism, and thyroid nodules (32, 33, 34). Furthermore, during 2001–2009/2015 a fall in threshold for treatment initiation of thyroid hormone therapy was observed in both Great Britain and in Denmark leading to more prescriptions of thyroid hormone therapy, but the fall in treatment threshold was markedly steeper in Denmark (35, 36). It is a limitation that we were unable to adjust for these between-country differences.
Overall, collection and comparisons of register-based data from European countries are time-consuming and complicated. When it comes to the burden of thyroid diseases, the potential consequences of differences in iodine intake status and IDD prevention programs may be overshadowed by within- and between-country sources of bias and confounding.
Differences in thyroid medication, surgery, and cancer rates
Despite the potential biases in comparisons, we still found fairly consistent rates of thyroid outcomes in line with previous research both when comparing the Scandinavian countries who have rather similar registers, healthcare systems, and health behaviors, but also when including data from quite different countries.
Thus, the findings of the highest rates of treatments most frequently applied for hyperthyroidism and/or structural thyroid diseases in countries with early iodine insufficiency and, conversely, the highest rates of treatment for hypothyroidism in countries with early iodine adequacy correspond with previous studies within countries comparing areas with high and low iodine intake (37, 38, 39) and over time when iodine adequacy was achieved after introduction of IDD prevention programs (40, 41, 42, 43). However, it is a limitation that the specific indication for treatment is not known.
We also found that rates of thyroid cancer did not correlate with early or recent iodine intake, for which previous results are not fully consistent (44). Previous studies have indicated that increased iodine intake is associated with an increase in the ratio between papillary and follicular thyroid cancer (45), which was not measured in our study. Nevertheless, whether the overall incidence of thyroid cancer is influenced by the population level iodine intake is still not clear (44).
We chose to only examine overall patterns in the relationship between iodine intake and rates of thyroid outcomes because the available data on iodine intake were collected by different methods, timepoints, and populations across the European countries and thus not sufficiently comparable. If valid data on early and recent iodine intake in Europe are harmonized it will be possible to perform more advanced statistical analyses of associations between the burden of thyroid diseases and iodine intake (46, 47).
Of note, our results indicate a higher correlation between early iodine intake and thyroid outcomes than recent iodine intake. Many of the included countries have introduced IDD prevention programs and achieved iodine sufficiency during the past two decades, and thus the adult population may still be affected by the exposure to decades of iodine insufficiency. Biologically it is plausible that thyroid function and thereby thyroid disease are influenced by the lifetime iodine intake level (48). Furthermore, the introduction of IF can cause a temporary rise in incident hyperthyroidism in countries with previous ID (8) or may induce thyroid dysfunction due to excessive iodine intake (49). This could affect the rates of treatment in several included countries. In Denmark, steady states were achieved for rates of thyrotoxicosis but not for hypothyroidism approximately 10 years after the implementation of mandatory IF (42, 43). Our study indicates that it may take even longer before the difference between former iodine adequacy and iodine insufficiency is negated between countries. Despite overall iodine adequacy, iodine deficiency can still be present in vulnerable groups, for example, pregnant women, which is observed in several countries (46).
The results show no differences between countries characterized by different IDD prevention strategies. Thus, a best-practice model of IDD prevention cannot be identified through this study. This may be explained, firstly, by the difference in coverage and impact of especially voluntary IF. Second, IDD prevention programs are optimally designed to compensate for the level of iodine deficiency in the target population, thus, similar iodine intakes may be achieved from different IDD prevention strategies.
Strengths and limitations
The main strengths of this study are, firstly, the strong collaboration between representatives from thyroid research and clinical environments from the European countries. The national EUthyroid representatives had or obtained knowledge of the availability and documentation of data that would not be possible for one researcher from one country. Second, the acquisition of several thyroid outcomes enabled us to evaluate different aspects of thyroid disease and the relationship with iodine intake. Third, the critical assessment of data secured that only data of appropriate quality was included. However, the critical assessment was based on evaluations of the available documentation, and thorough validation of most of the variables was not possible. Raw rates were calculated for all provided data included in the database, however, it is important to note that these should be interpreted with caution because the validity of some data is uncertain and comparison of the raw rates will be confounded by differences in sex- and age distributions. Only few variables met the criteria for comparison between countries and these variables were only available for few countries, which is a major limitation. This in combination with the heterogeneity of iodine intake data and lack of confounding factors limited the generalizability and our ability to draw more firm conclusions on the effect of iodine intake and IDD prevention programs.
This study elucidates the limitations of the registers and points out the need for greater participation and more uniformity in reporting in the future to optimally utilize the valuable registry information. The current increase in digitization and awareness of the possibilities within register research may lead to more valid register data sources on thyroid diagnoses and treatment with easy access and utilization in the European countries in the future. This will be of great value to thyroid research.
Conclusion and perspectives
The availability and quality of register data on thyroid diagnoses and treatments are highly heterogeneous between Europeans countries. Thus, comparison of rates of thyroid outcomes is currently only possible between a limited number of countries. The available data indicate lower rates of antithyroid medication and thyroid surgery for benign disease and higher rates of thyroid hormone therapy in countries with early iodine intake adequacy, and no difference for thyroid cancer or according to recent iodine intake.
As stated in the Krakow Declaration, evaluation of primary thyroid outcomes is essential in the control of IDD prevention programs (50). Registers may be an effective tool for this evaluation. Thus, the present project serves as initial exploration of the availability and quality of register data of thyroid outcomes across Europe, while more in-depth examination of the validity and comparability of registers is still needed to fully utilize the potential of the registers.
Supplementary Material
Declaration of interest
The authors have no conflicts of interest to declare. The author Alicja Hubalewska-Dydejczyk is an editor of the journal.
Funding
This work was supported by the European Union’s Horizon 2020 research and innovation program under grant agreement no. 634453. Data analyses were further supported by the project SUPERTHYREOSE, funded by the German G-BA (VSF2_2019-167). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mollehave LT, Linneberg A, Skaaby T, Knudsen N, Jorgensen T, Thuesen BH. Trends in treatments of thyroid disease following iodine fortification in Denmark: a nationwide register-based study. Clinical Epidemiology 201810763–770. ( 10.2147/CLEP.S164824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erlangsen A, Fedyszyn I. Danish nationwide registers for public health and health-related research. Scandinavian Journal of Public Health 201543333–339. ( 10.1177/1403494815575193) [DOI] [PubMed] [Google Scholar]
- 3.Forsea AM.Melanoma epidemiology and early detection in Europe: diversity and disparities. Dermatology Practical and Conceptual 202010 e2020033. ( 10.5826/dpc.1003a33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Bragt JJMH, Hansen S, Djukanovic R, Bel EHD, ten Brinke A, Wagers SS, Maitland-van der Zee AH, Porsbjerg C. SHARP: enabling generation of real-world evidence on a pan-European scale to improve the lives of individuals with severe asthma. ERJ Open Research 20217 00064-2021. ( 10.1183/23120541.00064-2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alriksson-Schmidt AI, Jeglinsky-Kankainen IFD, Jahnsen RB, Hollung SJ, Andersen GL, HÄgglund GV. Flaunting our assets. Making the most of the Nordic registry goldmine: cerebral palsy as an example. Scandinavian Journal of Public Health 202048113–118. ( 10.1177/1403494819829338) [DOI] [PubMed] [Google Scholar]
- 6.Siesling S, Louwman WJ, Kwast A, van den Hurk C, O’Callaghan M, Rosso S, Zanetti R, Storm H, Comber H, Steliarova-Foucher Eet al. Uses of cancer registries for public health and clinical research in Europe: results of the European Network of cancer registries survey among 161 population-based cancer registries during 2010–2012. European Journal of Cancer 2015511039–1049. ( 10.1016/j.ejca.2014.07.016) [DOI] [PubMed] [Google Scholar]
- 7.Lovestone SEMIF Consortium. The European medical information framework: a novel ecosystem for sharing healthcare data across Europe. Learning Health Systems 20204 e10214. ( 10.1002/lrh2.10214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet: Diabetes and Endocrinology 20153286–295. ( 10.1016/S2213-8587(1470225-6) [DOI] [PubMed] [Google Scholar]
- 9.Laurberg P, Cerqueira C, Ovesen L, Rasmussen LB, Perrild H, Andersen S, Pedersen IB, Carle A. Iodine intake as a determinant of thyroid disorders in populations. Best Practice and Research: Clinical Endocrinology and Metabolism 20102413–27. ( 10.1016/j.beem.2009.08.013) [DOI] [PubMed] [Google Scholar]
- 10.Schaffner M, Rochau U, Stojkov I, Qerimi Rushaj V, Völzke H, Marckmann G, Lazarus JH, Oberaigner W, Siebert U. Barriers against prevention programs for iodine deficiency disorders in Europe: a Delphi study. Thyroid 202131649–657. ( 10.1089/thy.2020.0065) [DOI] [PubMed] [Google Scholar]
- 11.WHO. Fortification of Food-Grade Salt with Iodine for the Prevention and Control of Iodine Deficiency Disorders. World Health Organization, 2014. [PubMed] [Google Scholar]
- 12.Völzke H, Caron P, Dahl L, de Castro JJ, Erlund I, Gaberšček S, Gunnarsdottir I, Hubalewska-Dydejczyk A, Ittermann T, Ivanova Let al. Ensuring effective prevention of iodine deficiency disorders. Thyroid 201626189–196. ( 10.1089/thy.2015.0543) [DOI] [PubMed] [Google Scholar]
- 13.Nystrom HF, Brantsaeter AL, Erlund I, Gunnarsdottir I, Hulthen L, Laurberg P, Mattisson I, Rasmussen LB, Virtanen S, Meltzer HM. Iodine status in the Nordic countries – past and present. Food and Nutrition Research 201660 31969. ( 10.3402/fnr.v60.31969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elvbakken KT, Meltzer HM. Research, knowledge, and policy on goitre and iodine in Norway (1850–2016). Centaurus 202163396–415. ( 10.1111/1600-0498.12371) [DOI] [Google Scholar]
- 15.Vandevijvere S, Mourri AB, Amsalkhir S, Avni F, Van Oyen H, Moreno-Reyes R. Fortification of bread with iodized salt corrected iodine deficiency in school-aged children, but not in their mothers: a national cross-sectional survey in Belgium. Thyroid 2012221046–1053. ( 10.1089/thy.2012.0016) [DOI] [PubMed] [Google Scholar]
- 16.Latvijas Vēstnesis. Regulation No. 696: regulations regarding edible salt. Riga, Latvia: : Latvijas Vēstnesis, 2015. [Google Scholar]
- 17.Global Fortification Data Exchange [updated 17th Feb 2021]. Iodine level. (available at: https://fortificationdata.org/map-nutrient-levels-in-fortification-standards/) [Google Scholar]
- 18.Trofimiuk-Müldner M, Konopka J, Sokołowski G, Dubiel A, Kieć-Klimczak M, Kluczyński Ł, Motyka M, Rzepka E, Walczyk J, Sokołowska Met al. Current iodine nutrition status in Poland (2017): is the Polish model of obligatory iodine prophylaxis able to eliminate iodine deficiency in the population? Public Health Nutrition 2020232467–2477. ( 10.1017/S1368980020000403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Iodine Status Worldwide: WHO Global Database on Iodine Deficiency. Geneva, Switzerland: World Health Organization, 2004. (available at: https://apps.who.int/iris/handle/10665/43010) [Google Scholar]
- 20.Global Scorecard of Iodine Nutrition (2014–2015) and (2020) Iodine Global Network. (available at: https://www.ign.org/scorecard.htm) [Google Scholar]
- 21.Vejbjerg P, Knudsen N, Perrild H, Carle A, Laurberg P, Pedersen IB, Rasmussen LB, Ovesen L, Jorgensen T. Effect of a mandatory iodization program on thyroid gland volume based on individuals’ age, gender, and preceding severity of dietary iodine deficiency: a prospective, population-based study. Journal of Clinical Endocrinology and Metabolism 2007921397–1401. ( 10.1210/jc.2006-2580) [DOI] [PubMed] [Google Scholar]
- 22.Mullan K, Hamill L, Doolan K, Young I, Smyth P, Flynn A, Walton J, Meharg AA, Carey M, McKernan Cet al. Iodine status of teenage girls on the island of Ireland. European Journal of Nutrition 2020591859–1867. ( 10.1007/s00394-019-02037-x) [DOI] [PubMed] [Google Scholar]
- 23.Ivanova LB.The prevention and control of iodine deficiency – achievements and problems in Bulgaria. Endocrinologia 20183121–131. [Google Scholar]
- 24.Zimmermann MB, Andersson M. Assessment of iodine nutrition in populations: past, present, and future. Nutrition Reviews 201270553–570. ( 10.1111/j.1753-4887.2012.00528.x) [DOI] [PubMed] [Google Scholar]
- 25.Revision of the European Standard Population – Report of Eurostat’s Task Force. Luxembourg: Publications Office of the European Union: European Commision, 2013. ( 10.2785/11470) [DOI] [Google Scholar]
- 26.Pacurariu A, Plueschke K, McGettigan P, Morales DR, Slattery J, Vogl D, Goedecke T, Kurz X, Cave A. Electronic healthcare databases in Europe: descriptive analysis of characteristics and potential for use in medicines regulation. BMJ Open 20188 e023090. ( 10.1136/bmjopen-2018-023090) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith Jervelund S, De Montgomery CJ. Nordic registry data: value, validity and future. Scandinavian Journal of Public Health 2020481–4. ( 10.1177/1403494819898573) [DOI] [PubMed] [Google Scholar]
- 28.Carle A, Bulow Pedersen I, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Jorgensen T, Laurberg P. Graves’ hyperthyroidism and moderate alcohol consumption: evidence for disease prevention. Clinical Endocrinology 201379111–119. ( 10.1111/cen.12106) [DOI] [PubMed] [Google Scholar]
- 29.Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Jørgensen T, Laurberg P. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: a population-based case-control study. European Journal of Endocrinology 2012167483–490. ( 10.1530/EJE-12-0356) [DOI] [PubMed] [Google Scholar]
- 30.Gruppen EG, Kootstra-Ros J, Kobold AM, Connelly MA, Touw D, Bos JHJ, Hak E, Links TP, Bakker SJL, Dullaart RPF. Cigarette smoking is associated with higher thyroid hormone and lower TSH levels: the PREVEND study. Endocrine 202067613–622. ( 10.1007/s12020-019-02125-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Kane SM, Mulhern MS, Pourshahidi LK, Strain JJ, Yeates AJ. Micronutrients, iodine status and concentrations of thyroid hormones: a systematic review. Nutrition Reviews 201876418–431. ( 10.1093/nutrit/nuy008) [DOI] [PubMed] [Google Scholar]
- 32.Burch HB, Burman KD, Cooper DS, Hennessey JV, Vietor NO. A 2015 survey of clinical practice patterns in the management of thyroid nodules. Journal of Clinical Endocrinology and Metabolism 20161012853–2862. ( 10.1210/jc.2016-1155) [DOI] [PubMed] [Google Scholar]
- 33.Burch HB, Burman KD, Cooper DS, Hennessey JV. A 2013 survey of clinical practice patterns in the management of primary hypothyroidism. Journal of Clinical Endocrinology and Metabolism 2014992077–2085. ( 10.1210/jc.2014-1046) [DOI] [PubMed] [Google Scholar]
- 34.Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. Journal of Clinical Endocrinology and Metabolism 2012974549–4558. ( 10.1210/jc.2012-2802) [DOI] [PubMed] [Google Scholar]
- 35.Taylor PN, Iqbal A, Minassian C, Sayers A, Draman MS, Greenwood R, Hamilton W, Okosieme O, Panicker V, Thomas SLet al. Falling threshold for treatment of borderline elevated thyrotropin levels-balancing benefits and risks: evidence from a large community-based study. JAMA Internal Medicine 201417432–39. ( 10.1001/jamainternmed.2013.11312) [DOI] [PubMed] [Google Scholar]
- 36.Medici BB, Nygaard B, La Cour JL, Grand MK, Siersma V, Nicolaisdottir DR, Lind B, Olivarius NF, Andersen CL. Changes in prescription routines for treating hypothyroidism between 2001 and 2015 – an observational study of 929,684 primary care patients in Copenhagen. Thyroid 201929910–919. ( 10.1089/thy.2018.0539) [DOI] [PubMed] [Google Scholar]
- 37.Du Y, Gao Y, Meng F, Liu S, Fan Z, Wu J, Sun D. Iodine deficiency and excess coexist in china and induce thyroid dysfunction and disease: a cross-sectional study. PLoS ONE 20149 e111937. ( 10.1371/journal.pone.0111937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen IB, Knudsen N, Jorgensen T, Perrild H, Ovesen L, Laurberg P. Large differences in incidences of overt hyper- and hypothyroidism associated with a small difference in iodine intake: a prospective comparative register-based population survey. Journal of Clinical Endocrinology and Metabolism 2002874462–4469. ( 10.1210/jc.2002-020750) [DOI] [PubMed] [Google Scholar]
- 39.Szabolcs I, Podoba J, Feldkamp J, Dohan O, Farkas I, Sajgó M, Takáts KI, Góth M, Kovács L, Kressinszky Ket al. Comparative screening for thyroid disorders in old age in areas of iodine deficiency, long-term iodine prophylaxis and abundant iodine intake. Clinical Endocrinology 19974787–92. ( 10.1046/j.1365-2265.1997.2271040.x) [DOI] [PubMed] [Google Scholar]
- 40.Baltisberger BL, Minder CE, Burgi H. Decrease of incidence of toxic nodular goitre in a region of Switzerland after full correction of mild iodine deficiency. European Journal of Endocrinology 1995132546–549. ( 10.1530/eje.0.1320546) [DOI] [PubMed] [Google Scholar]
- 41.Mostbeck A, Galvan G, Bauer P, Eber O, Atefie K, Dam K, Feichtinger H, Fritzsche H, Haydl H, Köhn Het al. The incidence of hyperthyroidism in Austria from 1987 to 1995 before and after an increase in salt iodization in 1990. European Journal of Nuclear Medicine 199825367–374. ( 10.1007/s002590050234) [DOI] [PubMed] [Google Scholar]
- 42.Petersen M, Knudsen N, Carle A, Andersen S, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB, Thuesen BH, Pedersen IB. Increased incidence rate of hypothyroidism after iodine fortification in Denmark: a 20-year prospective population-based study. Journal of Clinical Endocrinology and Metabolism 20191041833–1840. ( 10.1210/jc.2018-01993) [DOI] [PubMed] [Google Scholar]
- 43.Petersen M, Knudsen N, Carle A, Andersen S, Jorgensen T, Perrild H, Ovesen L, Rasmussen LB, Thuesen BH, Pedersen IB. Thyrotoxicosis after iodine fortification. A 21-year Danish population-based study. Clinical Endocrinology 201889360–366. ( 10.1111/cen.13751) [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann MB, Galetti V. Iodine intake as a risk factor for thyroid cancer: a comprehensive review of animal and human studies. Thyroid Research 20158 8. ( 10.1186/s13044-015-0020-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldt-Rasmussen U.Iodine and cancer. Thyroid 200111483–486. ( 10.1089/105072501300176435) [DOI] [PubMed] [Google Scholar]
- 46.Bath SC.The challenges of harmonising the iodine supply across Europe. Lancet: Diabetes and Endocrinology 20175411–412. ( 10.1016/S2213-8587(1630329-1) [DOI] [PubMed] [Google Scholar]
- 47.Ittermann T, Johner S, Below H, Leiterer M, Thamm M, Remer T, Völzke H. Interlaboratory variability of urinary iodine measurements. Clinical Chemistry and Laboratory Medicine 201856441–447. ( 10.1515/cclm-2017-0580) [DOI] [PubMed] [Google Scholar]
- 48.Chung HR.Iodine and thyroid function. Annals of Pediatric Endocrinology and Metabolism 2014198–12. ( 10.6065/apem.2014.19.1.8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farebrother J, Zimmermann MB, Andersson M. Excess iodine intake: sources, assessment, and effects on thyroid function. Annals of the New York Academy of Sciences 2019144644–65. ( 10.1111/nyas.14041) [DOI] [PubMed] [Google Scholar]
- 50.Völzke H.The Krakow declaration on iodine: tasks and responsibilities for prevention programs targeting iodine deficiency disorders. European Thyroid Journal 20187201–204. ( 10.1159/000490143) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Table 2 provides an overview of the data sources and Fig. 1 provides an overview of the flow of the data acquisition, critical assessments, and calculations.
Table 2.
Data requested and provided with stratification level and area.
| Country | Country code | Thyroid medication | Thyroid surgery | Radio-iodine | Fine needle biopsies | Diagnoses of thyroid disease | Diagnoses of thyroid cancer |
|---|---|---|---|---|---|---|---|
| Belgium | BE | Sex/age | Sex/age | Sex/age | Sex/age | N/A | Sex/age |
| Bulgaria | BG | Sex | Sex | Total | Sex | Sex | Sex/age |
| Croatia | HR | N/A | N/A | N/A | N/A | N/A | Sex |
| Czech Republic | CZ | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex |
| Denmark | DK | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Finland | FI | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Germany | DE | Sex/age a | N/A | N/A | N/A | Sex/agea | N/A |
| United Kingdom | UK | ||||||
| Northern Ireland | N/A | N/A | N/A | N/A | N/A | Sex/age | |
| Scotland | N/A | N/A | N/A | N/A | N/A | Sex/age | |
| Greece | GR | Totala | N/A | Totala | N/A | Totala | Totala |
| Hungary | HU | Sex/age | Sex/age | Sex/age | N/A | Age | Age |
| Iceland | IS | Sex | N/A | N/A | N/A | N/A | Sex |
| Ireland | IE | N/A | N/A | N/A | N/A | N/A | Sex/age |
| Israel | IL | Sex/age | N/A | N/A | N/A | Sex/age | Sex/age |
| Latvia | LV | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| North Macedonia | MK | Totala | N/A | N/A | N/A | N/A | N/A |
| Norway | NO | Sex/age | Sex/age | N/A | N/A | Sex/age | Sex/age |
| Poland | PL | Totala | Total | N/A | N/A | Total | Sex/age |
| Portugal | PT | Totala | Sex/age | Sex/agea | N/A | Sex/age | Sex/Age |
| Slovenia | SI | Total | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Spain | ES | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age | Sex/age |
| Sweden | SE | Sex/age | Sex/age | Total | N/A | Sex/age | Sex/age |
| Switzerland | CH | N/A | N/A | N/A | N/A | N/A | N/A |
Bold indicates National data; Italics indicate regional data or data from less defined sub-populations.
aData not suitable for inclusion in the database and calculation of raw rates.
N/A, not available.
Figure 1.
Overview of data availability, critical assessments, and calculation of rates.
Register data for at least one thyroid outcome was available and provided from 22 out of 23 participating countries/regions. However, the critical assessment concluded that data from three countries were considered unsuitable to be included in the database. See an overview of data and assessments in the Supplementary Tables 1 and 2 (see section on supplementary materials given at the end of this article). Raw rates for data included in the database are presented in the Supplementary Tables 3, 4, and 5.
The outcome variables were grouped into six categories: thyroid medication, thyroid surgery, radioiodine treatment, fine needle biopsies, diagnoses of thyroid diseases, and diagnoses of incident thyroid cancer. Sex- and age-stratified data from all categories were available from four countries, whereas data from only one outcome variable were available from four countries. The critical assessment of the data included in the database found that data on the use of thyroid medication, number of thyroid surgeries, and incidence of thyroid cancer were of acceptable quality and thus appropriate for calculation of standardized rates and comparison between countries.
In contrast, data on diagnoses of thyroid diseases except for thyroid cancer were not considered suitable for comparisons across countries. In some countries, registrations included patients treated in primary care whereas only patients attending hospitals were included in other countries, and it was not possible to distinguish between incidence and prevalence data. Furthermore, in some countries registration of diagnoses was based on the ICD-9 system whereas the ICD-10 system was used in other countries and the conversion of data from ICD-9 to ICD-10 caused some misclassification. Data on radioiodine and fine-needle biopsies were only available from a few countries and were generally considered unreliable.



 This work is licensed under a
This work is licensed under a