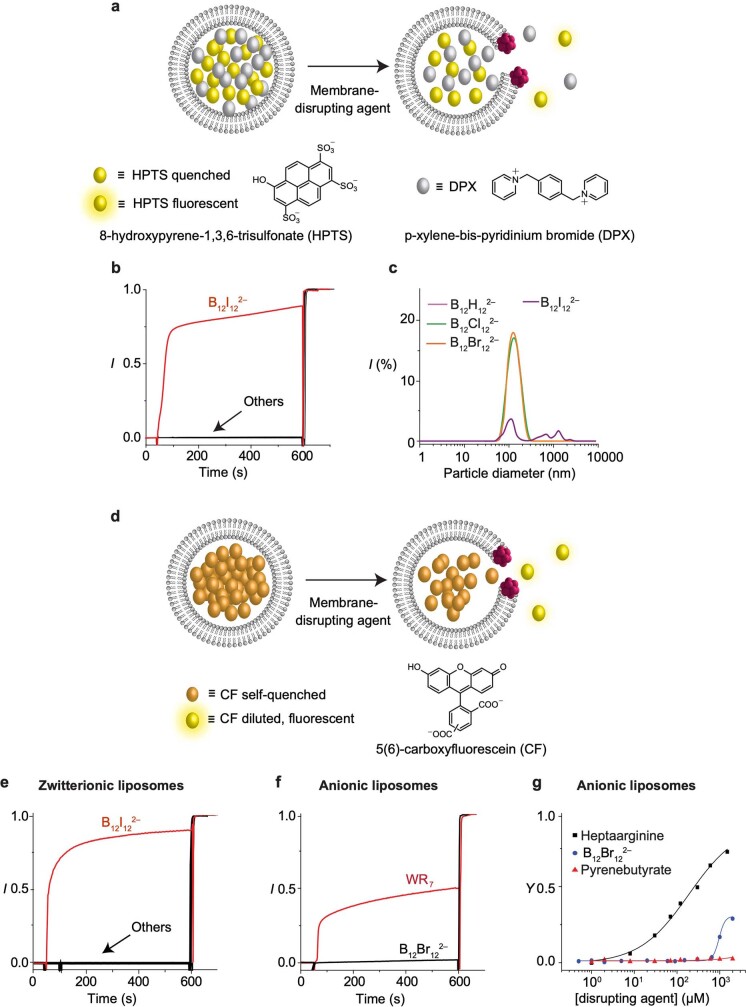
Extended Data Fig. 1. Assays testing for stability vs leakage of the vesicles.
a, Response of the HPTS/DPX assay towards a membrane-disrupting agent and chemical structures of the fluorescent dye HPTS and the quencher DPX. In this assay, large unilamellar vesicles are loaded with the HPTS/DPX probe/quencher pair, such that the fluorescence of HPTS is quenched. Although the addition of a membrane-compatible carrier causes only a response in the presence of suitable cargo (see Fig. 2 in main text) the addition of a membrane-disrupting agent (B12I122−) induces probe and quencher efflux, resulting in dilution with a concomitant sudden increase in fluorescence even in the absence of cargo. b, Changes in fractional HPTS emission intensity (λex = 413 nm, λem = 511 nm) of EYPC⊃HPTS/DPX vesicles (13 µM phospholipids in 10 mM Tris, 107 mM NaCl, pH 7.4) during the addition of 40 µM of clusters. The cluster B12I122− (red trace) was identified as lytic agent because the mere addition of the cluster led to a fluorescence increase. None of the other clusters (B12H122−, B12Cl122−, and B12Br122−) produced any perturbation in the vesicle membrane as monitored by a constant fluorescence intensity (baseline, black traces). c, Size distribution (by DLS) of EYPC⊃HPTS/DPX vesicles before and after addition of 200 µM cluster and 20 µM WR7 to differentiate the clusters that retain membrane integrity (B12H122−, B12Cl122−, and B12Br122−) from the one that lyses the vesicles (B12I122−). d, Working principle of the carboxyfluorescein (CF) leakage assay principle and chemical structure of the fluorescent probe. In this assay, large unilamellar vesicles are loaded with CF at high concentration, such that the fluorescence is quenched. The addition of a membrane-disrupting agent induces dye efflux, resulting in a CF dilution with the concomitant increase in fluorescence. e, Changes in fractional CF emission intensity (λex = 492 nm, λem = 517 nm) of EYPC⊃CF vesicles (13 µM phospholipids in 10 mM HEPES, 107 mM NaCl, pH 7.5) during the addition of 40 µM of clusters and 20 µM WR7. The cluster B12I122− (red trace) was identified as lytic agent because its mere addition led to a fluorescence increase. None of the other clusters (B12H122−, B12Cl122−, and B12Br122−) produced any perturbation in the vesicle membrane (even in the presence of WR7) as monitored by a constant fluorescence intensity (baseline). f, Changes in the fractional CF emission intensity (λex = 492 nm, λem = 517 nm) of DMPE/DPPG/CHOL⊃CF vesicles (13 µM phospholipids in 10 mM Tris, 140 mM NaCl, pH 7.4) on addition of 300 µM of WR7 (red trace) or B12Br122− (black trace), demonstrating lysis caused by the former and retained membrane integrity in the presence of the latter. g, Compatibility of the carrier B12Br122− (blue, up to ca. 1 mM) with anionic liposomes (DMPE/DPPG/CHOL 1/2/1) in comparison to the strongly membrane-disrupting WR7 peptide (black) and pyrenebutyrate (1, red). EC50 for WR7 = 190 ± 50 µM and B12Br122− = 960 ± 100 µM. In b, e and f, Triton X-100 was added at t = 600 s to induce vesicle lysis and allow data normalization.