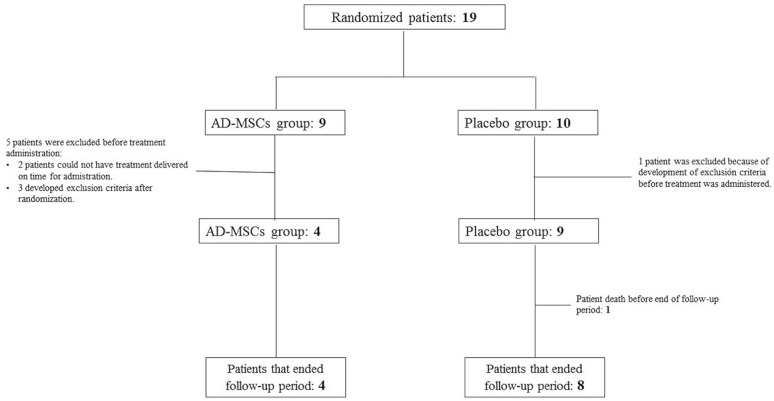
Figure 1.
AMASCIS final study sample. Trial profile. Nineteen patients were initially randomized: 9 to the AD-MSC group and 10 to the placebo treatment group. In the AD-MSC group, two patients did not receive the study treatment because it could not be delivered on time for administration (2 weeks since symptom onset), and three developed exclusion criteria after randomization and before study treatment was applied. In the placebo group, only one patient developed exclusion criteria before treatment administration. Finally, AD-MSCs were administered to four participants and placebo to eight. One of these eight patients died before the end of the follow-up period. AD-MSCs: adipose tissue–derived mesenchymal stem cells; AMASCIS: Allogeneic Adipose Tissue–Derived Mesenchymal Stem Cells in Acute Ischemic Stroke.