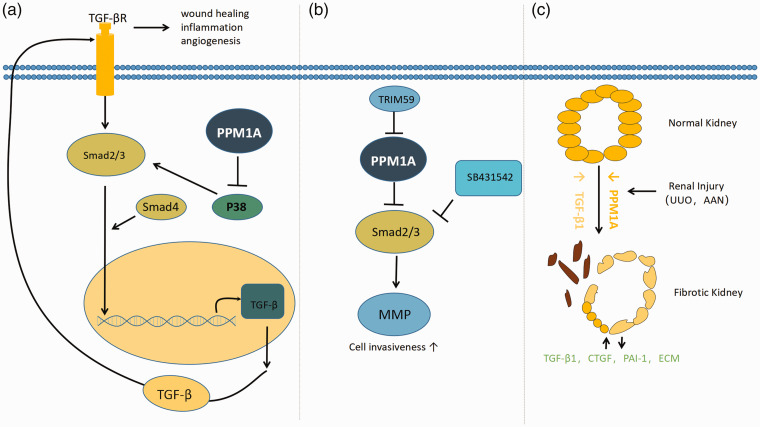
Figure 1.
Smad, as the substrate of PPM1A, regulates some diseases or physiological functions. (a) Model representing the role of PPM1A in the TGF-β signaling. PPM1A specifically dephosphorylates activated p38, making it easy to further phosphorylate in the presence of activation signal. However, p38 was over activated in the absence of PPM1A, resulting in enhanced transcription of TGF-related genes. TGF-β increased levels of activated TGF-β the receptor, in turn, activates its downstream Smad2/3, resulting in TGF-β. The lack of PPM1A in the adjusted cornea led to aberrant wound healing, inflammation, and neovascularization. (b) Model of Trim59 increases the invasion of endometrial stromal cells by ubiquitination of PPM1A. TRIM59 inhibits PPM1A through ubiquitination and activates TGF-β/Smad signaling to activate MMP (matrix metalloproteinase) and further promote the invasion of ectopic endometrial stromal cells in endometriosis. (c) Model for PPM1A involvement in renal fibrosis. PPM1A expression (orange) is readily evident in differentiated renal tubules. Kidney injury is accompanied by a progressive reduction (orange) of PPM1A in the tubular and interstitial regions, with increases in tissue TGF-b1 levels (yellow). Prolonged silencing of PPM1A promotes the secretion of PAI-1, CTGF, and extracellular matrix (ECM) and promotes the proliferation of fibroblasts through Smad3-dependent mechanism. (A color version of this figure is available in the online journal.)