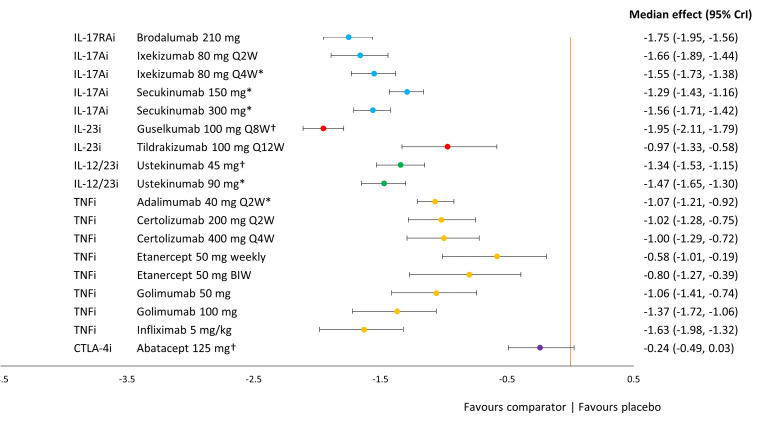
Figure 4.
Forest plot of treatment effects for key comparators versus placebo on PASI response. *Based on a combination of studies reporting outcomes at week 12 or 16 and week 24; †Based on studies reporting outcomes at week 24 only note that PASI treatment effect was based on a fixed-effects model with placebo adjustment. Median treatment effects and 95% credible intervals are plotted on the probit scale. Key comparators include bDMARDs at doses licensed for use in PSA or PSO. bDMARD, biological disease modifying antirheumatic drug; BIW, twice weekly; PASI, Psoriasis Area and Severity Index; PSA, psoriatic arthritis; PSO, psoriasis; Q2W, every two weeks; Q4W, every four weeks; Q8W, every eight weeks; Q12W, every twelve weeks.