Summary:
BMP signaling distinguishes between neural and non-neural fates by activating epidermis-specific transcription and repressing neural-specific transcription. The neural ectoderm forms after the Organizer secrets antagonists that prevent these BMP-mediated activities. However, it is not known whether neural genes also are transcriptionally activated. Therefore, we tested the ability of nine Organizer transcription factors to ectopically induce the expression of four neural ectodermal genes in epidermal precursors. We found evidence for two pathways: Foxd4 and Sox11 were only induced by Sia and Twn, whereas Gmnn and Zic2 were induced by Sia, Twn, as well as seven other Organizer transcription factors. The induction of Foxd4, Gmnn and Zic2 by Sia/Twn was both non-cell autonomous (requiring an intermediate protein) and cell autonomous (direct), whereas the induction of Sox11 required Foxd4 activity. Because direct induction by Sia/Twn could occur endogenously in the dorsal-equatorial blastula cells that give rise to both the Organizer mesoderm and the neural ectoderm, we knocked down Sia/Twn in those cells. This prevented the blastula expression of Foxd4 and Sox11, demonstrating that Sia/Twn directly activate some neural genes before the separation of the Organizer mesoderm and neural ectoderm lineages.
Keywords: Foxd5, Foxd4l1.1, Foxd4l1, Geminin, Sox11, Zic2, neural induction, Xenopus
INTRODUCTION
Establishment of the vertebrate neural ectoderm requires anti-BMP factors that are secreted by the Organizer mesoderm (reviewed in De Robertis and Kuroda, 2004; De Robertis, 2006; Itoh and Sokol, 2014; Khokha et al., 2005; Levine and Brivanlou, 2007; Wills et al., 2010). Repression of the BMP pathway enables the neural ectoderm to express numerous transcription factors that cooperatively solidify its neural fate (reviewed in Lee et al., 2014; Moody et al., 2013; Rogers et al., 2009; Sasai, 1998). The Organizer-derived diffusible factors, however, do not act directly on the neural genes to promote their transcription, but instead inhibit BMP receptor activation that leads to epidermis-specific transcription. For example, Vent transcription factors are activated by BMPs, up-regulate epidermal genes and directly repress Organizer and neural genes (Ault et al., 1997; Hoppler and Moon, 1998; Henningfeld et al., 2000; Imai et al., 2000; Onichtchouk et al., 1996, 1998; Ramel and Lekven, 2004; Rogers et al., 2008; Taylor et al., 2006; Yoon et al., 2014). An important question yet to be resolved is: are early neural genes expressed simply due to lack of binding of repressive regulators like the Vents, or are they directly activated as well?
To address this question, we screened a large number of Organizer transcription factors for their ability to ectopically induce neural genes in an epidermal lineage. These included: (1) Siamois (Sia), Twin (Twn), Goosecoid (Gsc), and Foxa4 (aka Pintalavis), which are expressed in both the dorsal-equatorial blastula cells that give rise to the Organizer and in the Organizer mesoderm itself; as well as (2) Xanf (aka Hesx1), Lim1, Xnot, Otx2, and XlPOU2 (aka Pou3f4), which are only expressed in the Organizer mesoderm and not its blastula precursors (Blitz and Cho, 1995; Cho et al., 1991; Gont et al., 1996; Kuroda et al., 2004; Laurent et al., 1997; Lemaire et al., 1995; Reversade and De Robertis, 2005; Ruiz i Altaba and Jessell, 1992; Wessely et al., 2004; Witta and Sato, 1997; Taira et al., 1994; Zaraisky et al., 1995). We were particularly interested in those factors expressed in dorsal-equatorial blastula cells for several reasons. First, maternal β-Catenin translocates into the nucleus of these cells to activate a dorsalizing program (Schneider et al., 1996). Second, molecular interactions occurring in these cells are required to form the Organizer mesoderm (Bae et al., 2011; Suduo et al., 2012). Third, these cells are necessary to form the nervous system (Ishibashi et al., 2008; Kuroda et al., 2004). Fourth, these cells are the common precursors of both the Organizer mesoderm and of the neural ectoderm (Bauer et al., 1994; Vodicka and Gerhart, 1995). Thus, if Organizer transcription factors were to directly interact with neural genes it would be in a cell autonomous manner in cells that give rise to both tissues.
To test for direct activation of neural genes we focused on early-expressed neural ectodermal (NE) genes. Epistasis experiments in Xenopus show that at least 12 NE genes interact in a regulatory network that controls the early formation and differentiation of the neural plate (Lee et al., 2014; Moody et al., 2013; Yan et al., 2009). A key upstream factor in this network is Foxd4 (aka Foxd5, Foxd4l1.1, Foxd4l1), whose zygotic expression begins at blastula stages, is down-regulated as the neural folds close, and is required for the expression of the 11 other genes (Fetka et al., 2000; Sölter et al., 1999; Sullivan et al., 2001; Yan et al., 2009). It also directly up-regulates three of them: Geminin (Gmnn), Zic2, and Sox11 (Yan et al., 2009). Together, these four transcription factors cooperatively maintain the NE in an immature, proliferative state, regulate its transition to neural plate stem cells, and delay neuronal differentiation (Sullivan et al., 2001; Yan et al., 2009).
In this study, we asked whether these four NE genes could be directly activated by Organizer transcription factors when ectopically expressed in epidermal precursors in the intact embryo. We found evidence for two pathways by which NE genes are activated: Foxd4 and Sox11 are ectopically induced only by Sia and Twn, whereas Gmnn and Zic2 are ectopically induced by Sia, Twn, as well as seven other Organizer transcription factors. The ectopic inductions of Sox11 by Sia and Twn were non-cell autonomous, indicating the necessity of an intermediate secreted factor. In contrast, the ectopic inductions of Foxd4, Gmnn and Zic2 were both non-cell autonomous and cell autonomous, the latter implicating direct transcriptional regulation. Direct transcriptional activation was confirmed by expressing hormone-inducible Sia and Twn constructs in the absence of protein synthesis. In these assays Sia directly activated Foxd4, both Sia and Twn directly activated Gmnn and Zic2, and neither directly activated Sox11. The cell autonomous inductions would require that the Organizer transcription factors activate the NE genes within the same cell before the separation of the Organizer mesoderm and NE lineages. Since fate map studies have identified such cells (Bauer et al., 1994; Vodicka and Gerhart, 1995), we knocked down Sia and Twn in the dorsal-equatorial region of the blastula and found that endogenous Foxd4 and Sox11 expression was eliminated. These results demonstrate that the blastula expression of Sia/Twn in cells that give rise to both the Organizer mesoderm and the NE can directly activate some NE genes before gastrulation.
RESULTS
Do Organizer Transcription Factors Ectopically Induce Early NE Genes?
In the blastula, the dorsal-equatorial cells that give rise to both the Organizer mesoderm and the NE (Bauer et al., 1994; Vodicka and Gerhart, 1995) are known to express four transcription factors (Sia, Twn, Gsc, Foxa4); in the gastrula, the Organizer mesoderm expresses these factors as well as Xanf (aka Hesx1), Lim1, Xnot, Otx2, and XlPOU2 (aka Pou3f4) (Blitz and Cho, 1995; Cho et al., 1991; Gont et al., 1996; Kuroda et al., 2004; Ishibashi et al., 2008; Laurent et al., 1997; Lemaire et al., 1995; Reversade and De Robertis, 2005; Ruiz i Altaba and Jessell, 1992; Suduo et al., 2012; Taira et al., 1994; Wessely et al., 2004; Witta and Sato, 1997; Zaraisky et al., 1995). To determine if these factors might directly regulate NE genes we ectopically expressed each one by mRNA injection into the 16-cell blastomere precursor of the ventral epidermis (Moody, 1987) at doses that previous publications determined were optimal for inducing ectopic Organizer and/or dorsal axial tissue or for altering dorsal axis formation. This assay tests induction in a region of the embryo that is devoid of Organizer or neural gene expression. Because it is a region subjected to endogenous anti-neural signaling (via BMP and Wnt) and epidermal transcriptional activity (via Vents), it also tests whether the ectopically expressed transcriptional program can over-ride the epidermal program.
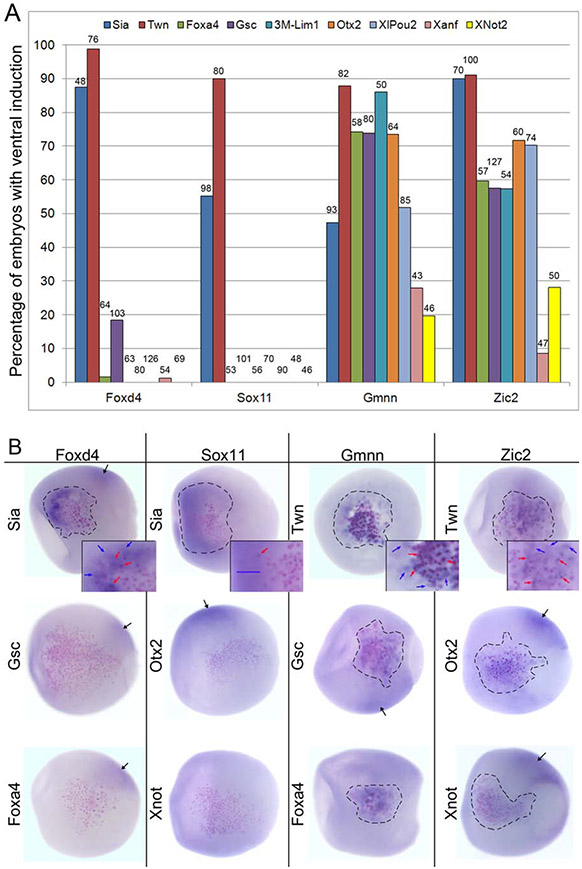
All four NE genes were ectopically induced at high frequencies by Sia and by Twn in gastrulae (stages 10.5-12.5; Fig. 1A). Some of the NE gene-expressing cells (blue arrows, Fig. 1B) were adjacent to Sia- and Twn-expressing cells that were marked by the nuclear βGal (nβGal) lineage tracer. This indicates non-cell autonomous induction via a diffusible factor, as would be expected for an Organizer transcription factor that acts indirectly on the ectoderm via secreted inhibitory factors. However, in addition, several Foxd4-, Gmnn- and Zic2-expressing cells were nβGal-positive, i.e., they were part of the Sia- or Twn-expressing clone of cells (red arrows, Fig. 1B). This suggests a cell autonomous induction within the same cell, indicating that Sia and Twn may also regulate Foxd4, Gmnn and Zic2 directly. Since Sox11 induction was only non-cell autonomous we conclude that its ectopic induction is only indirect via secreted factors.
FIG. 1.
NE genes are ectopically induced in the ventral epidermis by different sets of Organizer transcription factors. (a) The percentage of embryos in which an ectopic ventral patch of gene expression (Foxd4, Sox11, Gmnn, Zic2) was observed after mRNA encoding an Organizer transcription factor, indicated by colored bars (key is across the top), was injected into a ventral epidermis progenitor (V1.1; Moody, 1987). The number of embryos analyzed is shown over each bar. (b) Examples of ectopic ventral induction of the four NE genes in response to injection of Organizer transcription factor mRNAs. Induced clones are outlined in black. Foxd4 and Sox11 are ectopically induced by Sia, but not by Gsc, Otx2, Foxa4 or Xnot2, whereas Gmnn and Zic2 are ectopically induced by each of these genes. Images are of the ventral sides of gastrula stage embryos, animal pole to the left. The cells expressing the Organizer transcription factor (noted to the left of each embryo) are identified by pink nuclei (nbgal staining). The NE genes, labeled at the top of each column, were detected by ISH (purple reaction product). Higher magnification insets in the top row show examples of nβgal-positive cells that are also NE gene-positive (red arrows); for these cells the induction is likely cell autonomous. Blue arrows indicate cells that are nβgal-negative and NE gene-positive; for these cells the induction is non-cell autonomous. For Sox11, the blue bar indicates a broad region of ectopic induction in which there are no nβgal-positive cells, demonstrating that most of its induction is indirect. Black arrows indicate the endogenous expression domain of the NE gene on the dorsal side of the embryo.
Neither Foxd4 nor Sox11 were significantly induced by any other tested transcription factor; Gsc, which is a direct target of Sia (Bae et al., 2011; Kessler, 1997; Laurent et al., 1997; Reid et al., 2012), ectopically induced Foxd4 in only a few embryos. In contrast, Gmnn and Zic2 were ectopically induced at high frequencies by Gsc and Foxa4 (Fig. 1A), which are expressed in both the dorsal-equatorial blastula cells and in the Organizer mesoderm. They also were induced at high frequencies by Lim1, Otx2 and XlPOU2 and at lower frequencies by Xanf and Xnot2 (Fig. 1A). In most cases the ectopic inductions of Gmnn and Zic2 were both cell autonomous (in nβGal-positive cells; red arrows,) and non-cell autonomous (in adjacent nβGal-negative cells; blue arrows) (Fig. 1B). These results demonstrate that Foxd4/Sox11 and Gmnn/Zic2 are differentially induced by the nine Organizer transcription factors. The non-cell autonomous inductions are most likely due to the Organizer gene activating expression of diffusible factors, whereas the cell autonomous inductions likely result from direct activation within the same cell.
Is the Ectopic Induction of NE Genes by Sia/Twn Direct?
To test whether the four NE genes could be direct targets of Sia or Twn, we expressed hormone-inducible Sia (hGR-Sia) or Twn (hGR-Twn) mRNAs in the cleavage stage blastomere precursor of the ventral epidermis. The hGR fusion proteins are synthesized shortly after injection of the mRNA, but the hGR domain forms a complex with endogenous HSP90 in the cytoplasm, preventing them from accessing the nucleus (Kolm and Sive, 1995; Mattioni et al., 1994). Embryos were treated with cyclohexamide (Chx) to block protein synthesis at blastula stages 8-8.5 (Cho et al., 1991), and treated 40 minutes later with the synthetic hormone dexamethasone (Dex) to uncouple the complex and allow nuclear translocation of the hGR-transcription factor. To ensure that the fusion protein was active, hGR-Sia-injected or hGR-Twn-injected embryos were treated only with Dex at either cleavage or blastula stages (Fig. 2A-C); for all four NE genes the ventral induction was as strong as that of the wild type constructs.
FIG. 2.
NE genes are directly induced in the ventral epidermis by Sia or Twn. (a) The percentage of embryos in which an ectopic ventral patch of gene expression (Foxd4, Sox11, Gmnn, Zic2) was observed after injection of hGR-Sia mRNA. Embryos were either not treated with hormone (no dex, blue bars), treated with hormone at the 64-cell cleavage stage (dex-CL, red bars), treated with hormone at the stage 8 blastula (dex-BL, green bars), or pretreated at stage 8 with a protein synthesis inhibitor 40 minutes before hormone treatment (Chx+dex, purple bars). The high frequency of induction of ectopic gene expression with dex treatment alone at either cleavage or blastula stages indicates that the construct is hormone-inducible. Foxd4, Gmnn and Zic2 are likely direct targets of Sia because in the presence of Chx they are induced at the same frequency as dex treatment alone, and at a significantly greater frequency compared to no dex embryos (*,p < 0.001); Sox11 is not a direct target because it is not induced when protein synthesis is blocked by Chx. The number of embryos analyzed is shown over each bar. (b) The percentage of embryos in which an ectopic ventral patch of gene expression (Foxd4, Sox11, Gmnn, Zic2) was observed after injection of hGR-Twn mRNA. The data are presented as described in A. Gmnn and Zic2 are likely direct targets of Twn (*,p < 0.001), whereas Foxd4 and Sox11 are not. The number of embryos analyzed is shown over each bar. (c) When an embryo is injected with Sia-hGR mRNA and not treated with Chx or dex (Chx-/dex−) (left image), NE genes (in this case Foxd4) are not induced; the Sia-hGR expressing cells (outlined, and in the inset) have pink nuclei but no purple reaction product. When an embryo is injected with Sia-hGR or Twn-hGR mRNA and treated with only dex (Chx-/dex+), NE genes are induced (outlined). For Sox11, there is considerable non-cell autonomous induction (in area indicated by blue arrow), whereas for Gmnn nearly all the labeled cells have pink nuclei (inset). Black arrows indicate endogenous expression domain. (d) Examples of direct induction of NE genes by hGR-Sia or hGR-Twn mRNAs. The injected mRNAs (Sia-hGR, Twn-hGR) are indicated to the left of each image, and the assayed NE gene is indicated at the upper left of each row. Embryos treated only with Chx do not show ectopic induction (Chx+/dex−); only pink nuclei are visible. Two examples of responses to Sia-hGR and one example of responses to Twn-hGR direct induction at blastula stages (Chx+/dex+) are shown for Foxd4, Sox11, and Gmnn; two examples of responses to Twn-hGR and one example of response to Sia-hGR direct induction at blastula stages (Chx+/dex+) are shown for Zic2. The region of ectopic induction (purple) is outlined by red dashes in each case. Sia directly induces Foxd4, Gmnn and Zic2; Twn directly induces Gmnn and Zic2. Sox11 is not directly induced by either.
Both Foxd4 and Sox11 were ectopically induced after Dex treatment at high frequency by both constructs in early gastrulae (stages 10.5-11.5; Fig. 2A-C). In embryos incubated in Chx before Dex treatment, however, Foxd4 was induced only by hGR-Sia; the frequency of induction was comparable to Dex treatment alone at either cleavage or blastula stages and significantly higher than in embryos not treated with Dex (Fig. 2A,D). In these cases only cells belonging to the Sia-expressing clone (nβGal-positive) expressed Foxd4 (Fig. 2D), indicating only a cell autonomous induction. Chx treatment alone did not induce Foxd4 (0/28 embryos), indicating that Chx was not indirectly preventing the activity of a repressor (Kurth et al., 2005). Interestingly, Foxd4 was not induced by hGR-Twn in Chx+Dex-treated embryos. This indicates that Twn homodimers cannot directly activate Foxd4. In contrast, Sox11 was not induced in Chx+Dex-treated embryos by either hGR-Sia or hGR-Twn (Fig. 2A,B,D). This is consistent with the lack of cell autonomous induction by the wild-type proteins (Fig. 1B), and indicates that the Sia-mediated induction of Sox11 requires an intermediate protein.
Both Gmnn and Zic2 were ectopically induced after Dex treatment at high frequency by both constructs (Fig. 2A,B,D). In embryos incubated in Chx before Dex treatment, they both were induced by both hGR-Sia and hGR-Twn; the frequency of induction was comparable to Dex treatment alone at either cleavage or blastula stages and significantly higher than in embryos not treated with Dex (Fig. 2A-D). In these cases, only cells belonging to the Sia-positive or Twn-positive clone (nβGal-positive) expressed Gmnn or Zic2 (Fig. 2D). Neither Gmnn nor Zic2 was induced by Chx treatment alone in either hGR-Sia (Gmnn, 0/28; Zic2, 0/24) or hGR-Twn (Gmnn, 0/15; Zic2, 0/16) injected embryos. These results confirm that Gmnn and Zic2 are not indirectly induced by inhibiting the synthesis of repressors (Kurth et al., 2005), but instead are directly activated by both Sia and Twn.
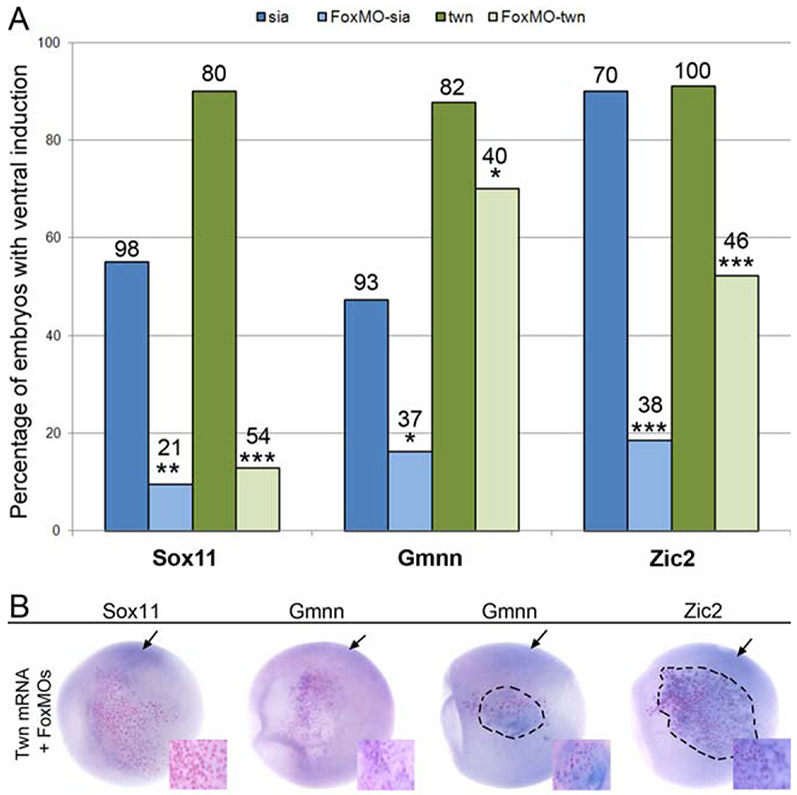
Previously we reported that Sox11, Gmnn and Zic2 expression in the neural ectoderm of the gastrula requires Foxd4, and that they each can be directly activated by Foxd4 (Yan et al., 2009). Since Sia and Twn also induce each of these NE genes, we tested whether Foxd4 activity is required. First, we injected Foxd4 anti-sense morpholino oligonucleotides (FoxMOs), which previously were shown to effectively block Foxd4 translation (Yan et al., 2009), into one 8-cell ventral animal blastomere. Next, one of the 16-cell daughter blastomeres was injected with wild-type Sia or wild-type Twn mRNA. The ectopic expression of Sox11 by either Sia or Twn was nearly eliminated in these embryos (Fig. 3A,B), indicating that Foxd4 mediates the induction of Sox11 by these two Organizer genes. Foxd4 knock-down also strongly reduced the Sia-mediated induction of Gmnn and Zic2, but the effects on Twn-mediated induction were much weaker, albeit statistically significant (Fig. 3A,B). Cells receiving only FoxMOs also did not ectopically express NE genes (0/74, Sox11; 0/73, Gmnn; 2/76, Zic2) These results indicate that Foxd4 activity is required for Sia induction of Gmnn and Zic2, but only moderately facilitates their Twn-mediated induction.
FIG. 3.
Foxd4 mediates some of the ectopic induction of Sox11, Gmnn and Zic2. The percentage of embryos in which an ectopic patch of gene expression (Sox11, Gmnn, Zic2) was observed after injection of Sia (blue bars) or Twn (green bars) mRNA in a ventral epidermal lineage in which Foxd4 translation was knocked-down (FoxMO). Sox11 induction by Sia and Twn was dramatically reduced in the absence of Foxd4. Gmnn and Zic2 induction by Sia was more strongly affected by the absence of Foxd4 than was their induction by Twn (***,p < 0.001;**, p < 0.01;*, p < 0.05). The number of embryos analyzed is shown over each bar. Examples of ectopic ventral expression of Sox11, Gmnn and Zic2 in response to injection of Twn mRNA in the presence of Foxd4 MOs. Black arrows point to the endogenous expression domains of the genes on the dorsal side. In most cases, there was no detectable Sox11 expression at the site of Twn expression (pink nuclei without purple reaction product; inset). For Gmnn and Zic2, some embryos showed no induction in the absence of Foxd4 (Gmnn left embryo; pink nuclei without purple reaction product; inset), whereas in others there was robust induction despite the knock-down of Foxd4 (Gmnn, right embryo and Zic2; pink nuclei surrounded by purple reaction product; insets). All embryos are oriented with animal pole to left and vegetal pole to right.
Is the Expression of NE Genes Initiated in the Blastula?
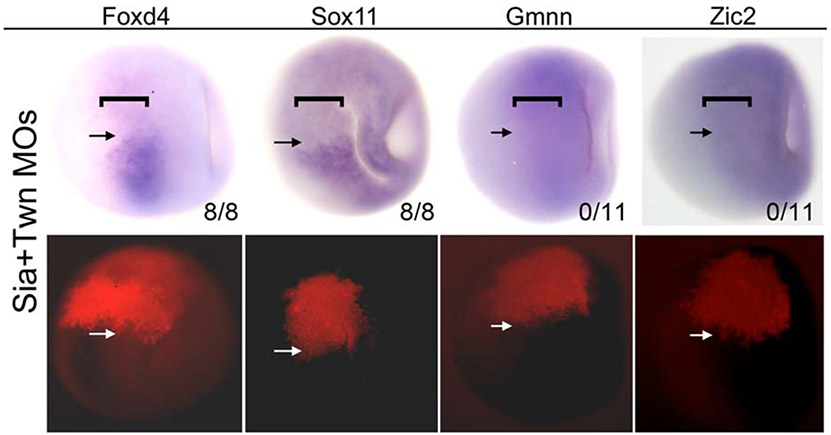
The ectopic induction assays indicate that Sia and Twn induce Foxd4, Gmnn and Zic2 expression both indirectly, i.e., in adjacent cells, and directly, i.e., in the same cell. The indirect regulation likely occurs via the well characterized anti-BMP and anti-Wnt neural inductive signaling from the Organizer mesoderm to the adjacent ectoderm. However, since direct regulation must occur within the same cell we hypothesized that this interaction could occur within a dorsal-equatorial blastula precursor that gives rise to both the Organizer mesoderm and the NE. This seemed possible because these cells are required for both Organizer and neural development and they express both Organizer genes as well Foxd4, Gmnn and Zic2 (Fig. 4). To test this, we used MOs to knock down both Sia and Twn in the dorsal-equatorial blastula cells on one side of the embryo; both proteins need to be knocked-down to observe a phenotype due to redundancy in DNA binding (Bae et al., 2011; Reid et al., 2012). The blastula expression of Foxd4 and Sox11 was eliminated in the MO-containing cells as early as stage 9, whereas Gmnn or Zic2 expression was not detectably altered (Fig. 4). These results indicate that zygotic expression of Foxd4 is initiated in the blastula by Sia/Twn, which in turn is required for Sox11 expression before gastrulation. They also indicate that Gmnn and Zic2 may not require Sia/Twn activation in the blastula.
FIG. 4.
Sia/Twn are required for blastula expression of Foxd4 and Sox11. Paired bright field (top row) and fluorescence (bottom row) images of blastula stage embryos in which Sia+Twn MOs were injected into a 16-cell blastomere that gives rise to the dorsal-equatorial blastula precursors of the Organizer mesoderm and neural ectoderm (marked by a bracket in the top row). The location of the MOs is visualized by red (lissamine) fluorescence on the left side of the embryo (above the white arrows in the bottom row). Normal Foxd4 and Sox11 expression is more restricted to these dorsal-equatorial cells (brackets), whereas Zic2 and Gmnn normally are expressed throughout the animal hemisphere including the dorsal-equatorial cells (shown in right side of each embryo; below the black arrows). Foxd4 and Sox11 expression is greatly reduced in the region containing Sia/Twn MOs (above the black arrows in the top row). In contrast, Gmnn and Zic2 expression appears unaffected. The numbers refer to the number of embryos in which gene expression was repressed by Sia/Twn knock down. Dorsal views of embryos are oriented with animal pole to the right.
DISCUSSION
Foxd4 is transiently expressed from blastula stages to the closure of the neural tube, and plays a key role in the early steps of NE development (Fetka et al., 2000; Sölter et al., 1999; Sullivan et al., 2001; Yan et al., 2009). We previously showed that Foxd4 is a critical upstream regulator of a NE regulatory network (Lee et al., 2014; Moody et al., 2013; Yan et al., 2009). Decreasing the level of Foxd4 in the NE reduced the expression of 11 other early neural genes. Increasing the level of Foxd4 up-regulated Gmnn and Zic2, which promote a proliferative, immature neural ectoderm (Brewster et al., 1998; Kroll, 2007; Kroll et al., 1998; Seo et al., 2005; Seo and Kroll, 2006), and up-regulated Sox11, which transitions immature neural progenitors towards differentiation (Bergsland et al., 2006; Hyodo-Miura et al., 2002; Uwanogho et al., 1995). Furthermore, Foxd4 repressed BMP gene transcription and BMP signaling (Yan et al., 2009, 2010). Thus, Foxd4 plays a very early role in establishing the nascent NE and preventing its conversion to a non-neural fate. Its central and early role provided a unique opportunity to examine whether NE genes are activated directly.
The studies reported here provide the first evidence that the earliest-expressed NE genes are activated by the maternal β-Catenin targets, Sia and Twn, that their activation is direct, and that this activation begins in the blastula. Firstly, Foxd4, Sox11, Gmnn, and Zic2 were ectopically induced by expressing Sia and Twn in ventral epidermal precursors. The evidence for direct activation is that Foxd4, Gmnn and Zic2 are induced by Sia even when protein synthesis is inhibited, and that Gmnn and Zic2 are induced by Twn following protein synthesis inhibition. Additionally, induction of Foxd4, Gmnn and Zic2 was observed in both the cells expressing Sia/Twn and in neighboring cells following ventral injection of Sia/Twn mRNA, whereas this induction was observed only in the same cell when protein synthesis was blocked. Finally, the blastula expression of Foxd4 and Sox11 was prevented by knocking down of Sia/Twn in the dorsal-equatorial blastula cells that give rise to both the Organizer mesoderm and the NE. Taken together, these observations show that Sia/Twn can directly activate some NE genes in the blastula.
While several signaling pathways, e.g. FGF and Wnt, also play important roles in the process of neural induction (Fletcher et al., 2006; Mulligan and Cheyette, 2012; Pera et al., 2014; Pinho et al., 2011; Rogers et al., 2011; Stern, 2006; Streit et al., 2000; Young et al., 2014), it is well established in several non-mammalian vertebrates that an essential step in inducing the neural ectoderm is the inhibition of BMP signaling by Organizer activity. Recent studies in invertebrates and in mouse ESCs and embryos confirm the critical role of inhibiting BMP signaling (Dang et al., 2012; Kozmikova et al., 2013; Li et al., 2013). Key transcriptional regulators of Organizer activity are Sia and Twn, two highly related proteins that directly activate the transcription of a number of genes required for initiating neural induction via secreted anti-BMP and anti-Wnt factors. However, there has not previously been evidence that Organizer genes directly up-regulate neural genes. Herein we demonstrate in an ectopic expression assay in the intact embryo that Sia and Twn can directly activate three genes that are important for establishing the NE, and that at least one of these, Foxd4, is activated in the blastula before the separation of the Organizer mesodermal and NE lineages. It is of interest that DUXO, a double-homeobox transcription factor that is unique to placental mammals and bears high homology to Sia and Twn in its homeodomains, also is required for Organizer gene expression in human embryonic stem cell cultures (Sharon et al., 2012), suggesting that there may be conservation of our findings with mammals.
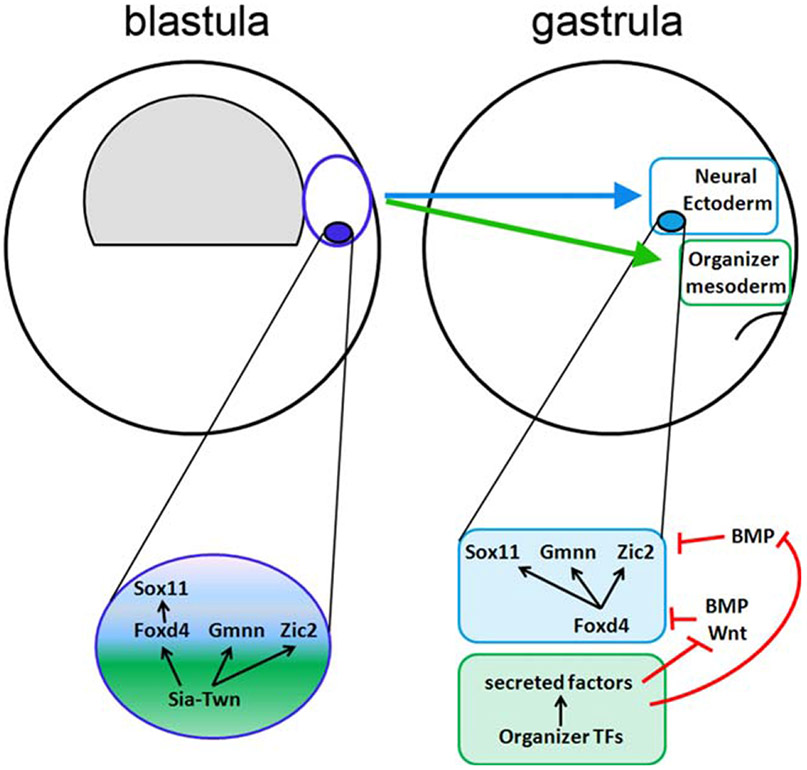
From these observations we propose that two pathways regulate the expression of early NE genes (Fig. 5). First, within dorsal-equatorial blastula cells, Sia directly activates Foxd4 expression, which in turn activates Sox11. Sia and Twn may directly activate Gmnn and Zic2 as well (see below), but we were not able to demonstrate this due to the high levels of maternal transcripts. Then, at gastrulation, after dorsal-equatorial blastula lineages have segregated into Organizer mesoderm and NE, Sia and Twn activity in the Organizer up-regulates a number of other transcription factors, leading to the secretion of inhibitory factors. These in turn diffuse into the adjacent ectoderm to prevent BMP- and Wnt-mediated repression of NE genes, and thereby maintain the expression of NE genes.
FIG. 5.
Summary of Sia/Twn time and location of induction of NE genes. At the blastula stage, cells that are fated to give rise to both Organizer mesoderm and neural ectoderm express Sia and Twn, which in turn directly activate Foxd4, Gmnn and Zic2; Sox11 activation requires the presence of Foxd4. At the gastrula stage, when the descendants of these cells have segregated into the Organizer mesoderm and the neural ectoderm, NE genes are up-regulated indirectly by Organizer transcription factors that regulate the expression of secreted factors that inhibit the BMP and Wnt pathways. Previous studies show that Sox11, Gmnn and Zic2 are expressed by inhibiting BMP, whereas Foxd4 is best expressed when both BMP and Wnt signaling pathways are blocked (refs in text). In the neural ectoderm, Foxd4 directly activates Sox11, Gmnn and Zic2 (Yan et al., 2009).
Interestingly, Sia/Twn knock-down did not affect the blastula expression of Gmnn or Zic2 even though they appear to be direct targets in the ectopic expression assay. There are two plausible explanations for this apparent discrepancy. First, Sia/Twn may not directly induce these two genes in the endogenous environment, whereas in the ectopic induction assay (Fig. 1) they may have an experimentally provided opportunity to bind to the enhancer/promoter regions of these NE genes. Alternatively, the bulk of the Gmnn and Zic2 mRNAs detected at blastula stages is likely of maternal origin, which would not be affected by Sia/Twn MO knock-down in the blastula. We favor this interpretation because Gmnn and Zic2 are reported to have strong maternal expression (Houston and Wiley, 2005; Kroll et al., 1998), and RNA-Seq data confirm that they have high levels of maternal expression from cleavage through blastula stages (Collart et al., 2014; Yanai et al., 2011). In contrast, Foxd4 maternal mRNA is of low abundance and its zygotic mRNA levels significantly increase at blastula stages slightly after the zygotic expression of Sia (Collart et al., 2014; Yanai et al., 2011). By mining the data from a high resolution time-line of Xenopus transcription at blastula stages (Collart et al., 2014), we found that Foxd4, Gmnn and Zic2 all show a transient uptick in mRNA levels just after the onset of Sia transcription (Yanai et al., 2011), and slightly in advance of a similar uptick in zygotic Sox11 mRNA levels (Collart et al., 2014). These temporal data are consistent with a model in which Sia and Twn directly activate Foxd4, Gmnn and Zic2 in the blastula, and Foxd4 directly activates Sox11 (Fig. 5). However, using ChIP-Seq approaches will be needed to convincingly demonstrate this course of events.
Consistent with our previous epistasis studies of NE genes (Yan et al., 2009), Sox11 induction in the blastula requires Foxd4 activity. In contrast, Gmnn and Zic2 can be directly activated by both Sia and Twn, and only the Sia-induction is reduced in the absence of Foxd4. Since Gmnn and Zic2 also can be ectopically induced by several Organizer-specific transcription factors, we conclude that they are regulated by multiple inputs. The Organizer transcription factors are not likely to act directly in the normal setting of the gastrulating embryo, but likely require intermediary secreted factors (Fig. 5). In fact, Gmnn and Zic2 are known to be highly induced ectopically simply by expressing anti-BMP factors (Brewster et al. 1998; Kroll et al., 1998).
Sia and Twn have key regulatory roles in activating Organizer and neural genes. Several studies show that Sia is required during blastula stages for the formation of the Organizer and for the expression of several Organizer-specific genes (Darras et al., 1997; Fan and Sokol, 1997; Kessler, 1997; Kodjabachian and Lemaire, 2001). It can activate Chordin, Noggin and Cerberus independent of Nodal signaling and mesoderm formation (Agius et al., 2000; Carnac et al., 1996; Darras et al., 1997; Ding et al., 1998; Kodjabachian and Lemaire, 2001; Medina et al., 1997), but Nodal signaling can enhance Sia induction of these genes (Crease et al., 1998; Darras et al., 1997; Engleka and Kessler, 2001) by enhancing its occupancy on their promoter/enhancer elements (Reid et al., 2012). Twn is highly related to Sia (Laurent et al., 1997; Nishita et al., 2000). Together with Sia it directly activates Gsc, Chd, and Cerb (Bae et al., 2011; Kessler, 1997; Laurent et al., 1997; Reid et al., 2012; Yamamoto et al., 2003). Several lines of evidence indicate that Twn and Sia redundantly activate Organizer genes. They bind to identical elements in the Gsc promoter, and they form homo- and heterodimers that bind equally well to this promoter (Bae et al., 2011). In certain assays there are no functional differences between the different types of dimers, and both proteins must be knocked down to completely eliminate Gsc, Chd, and Zic1 expression (Bae et al., 2011). However, we detected differences in Sia versus Twn effects on the ectopic induction of the four NE genes. While both Sia and Twn could ectopically induce all four NE genes without protein synthesis, Foxd4 was directly induced only by Sia, whereas Gmnn and Zic2 were directly induced by both Sia and Twn. These differences suggest that there is some specificity in the regulation of the different NE genes that was not observed for Gsc, Chd and Zic1, which are likely context-dependent. Our evidence indicates that Foxd4 can be induced by Sia homo-dimers but not by Twn homo-dimers, whereas Gmnn and Zic2 can be induced by both. The causes of these differences need to be resolved by DNA-binding assays, and by considering the roles of other proteins, such as XIC (Snider and Tapscott, 2005), that modify Sia induction of Organizer genes.
In summary, our results show that the Organizer transcription factors, Sia and Twn, can activate some NE genes at blastula stages. Sia directly activates Foxd4, which in turn activates Sox11. Once descendants of dorsal-equatorial blastula cells diverge into the Organizer mesoderm and NE lineages, Sia maintains NE genes indirectly via secreted factors. Although our experiments indicate that Gmnn and Zic2 also can be directly activated by Sia/Twn in a cell autonomous fashion, which most likely occurs in the blastula, we could not directly confirm this by Sia/Twn knock-down because Gmnn and Zic2 maternal mRNAs are very abundant. However, they do appear to be direct transcriptional targets of Twn, whereas their Sia induction is likely mediated by Foxd4 activity. In addition, our data are consistent with Gmnn and Zic2 later being indirectly activated in the neural ectoderm downstream of several Organizer transcription factors via secreted factors. Thus, the experiments described herein demonstrate that Sia/Twn activity in the blastula cells that give rise to both the Organizer mesoderm and the NE directly activates some NE genes, initiating neural fate before gastrulation.
METHODS
mRNA Synthesis and Injection.
To generate a hormone-inducible Twn plasmid, the Twn ORF with a StuI site at its 5′ end and an XhoI site at its 3′ end was made using standard PCR methods and cloned into pCS2+-hGR-MT using the StuI and XhoI sites in the polylinker. The pCS2+-hGR-MT-Twn plasmid was confirmed by sequencing and used as template to generate mRNA (hGR-MT-Twn). mRNAs encoding Foxa4 (500 pg/nl; Ruiz i Altaba and Jessell, 1992), Foxd4 (100 pg/nl; Sullivan et al., 2001), Goosecoid (Gsc; 500 pg/nl; Cho et al., 1991), an activated form of Lim1 (3MLim1, 750 pg/nl; Taira et al., 1994), Xnot2 (100 pg/nl; Gont et al., 1996), Otx2 (100 pg/nl; Blitz and Cho, 1995), XlPOU2 (200 pg/nl; Witta and Sato, 1997), Siamois (Sia; 150 pg/nl; Lemaire et al., 1995), hormone-inducible Siamois (hGR-Sia; 150 pg/nl; Kodjabachian and Lemaire, 2001), Twin (Twn; 5-10 pg/nl; Laurent et al., 1997), hormone-inducible Twin (hGR-Twn; 200 pg/nl), and Xanf (50 pg/nl; Zaraisky et al., 1995) were synthesized in vitro (Ambion, mMessage mMachine kit). The dose of each mRNA used was determined from previous publications as being optimal for inducing a secondary axis or specific dorsal axial structure, indicating induction of ectopic Organizer tissue, or for altering dorsal axis formation. These mRNAs were mixed with nuclear-localized βgal mRNA (nbgal, 100 pg/nl) as a lineage tracer and microinjected at published optimal concentrations in embryos that were obtained, cultured and microinjected as previously described (Moody, 1999, 2000). One nl of each mRNA mixture was microinjected into one ventral animal blastomere of the 16-cell embryo that is a defined precursor of the epidermis (blastomere V1.1; Moody, 1987). The resulting embryos were examined by in situ hybridization (ISH) at stages when early NE genes are highly expressed (stages 11.0–12.5; Nieuwkoop and Faber, 1994) to observe the spatial relationship between the injected progeny and the cells expressing Foxd4, Sox11, Gmnn or Zic2. The uninjected side of the embryo was used as an internal control.
Morpholino Knock-Down of Foxd4 in Combination With Sia/Twn mRNA Injection.
To determine if Foxd4 is required for Sia- or Twn-mediated ectopic induction, we performed sequential injections of Foxd4 antisense morpholino oligonucleotides (MOs) and then Sia or Twn mRNAs. A mixture of two Foxd4 MOs (20 ng), which were lissamine-tagged to visualize the injected progeny and targets both alloalleles to effectively prevent mRNA translation (Yan et al., 2009), were injected into a single ventral animal blastomere of the 8-cell embryo. After the next cell division was completed, its midline daughter (blastomere V1.1) was injected with either Sia or Twn mRNA, mixed with nβgal mRNA as a lineage tracer. The resulting embryos were examined at stages when early NE genes are highly expressed (stages 11.0–12.5).
Morpholino knock-down of Sia and Twn expression.
The endogenous levels of Sia and Twn were knocked down by injecting previously characterized MOs (9 ng; Bae et al., 2011), labeled with lissamine, into the 16-cell common progenitor of the Organizer mesoderm and NE (blastomere D1.1; Bauer et al., 1994; Moody, 1987) on one side of the embryo. The resulting embryos were examined at late blastula (stage 9).
Hormone induced expression and cycloheximide treatment.
hGR-Sia or hGR-MT-Twn mRNA, mixed with nbgal mRNA as lineage tracer, was injected into one ventral animal blastomere of the 16-cell embryo. Injected embryos were incubated in synthetic hormone (10 μM dexamethasone, Dex) according to published protocols (Kolm and Sive, 1995). To ensure that the hGR-Sia and hGR-MT-Twn constructs functioned as expected, some injected embryos were treated with hormone immediately after mRNA injection or at blastula stage 9. These embryos phenocopied those injected with wild-type mRNAs. Some embryos were injected with hGR mRNAs and raised in the absence of hormone; ectopic induction of target genes was observed in fewer than 10% of these embryos, indicating that the hormone-inducible constructs had little effect in the absence of hormone, in accord with published accounts (de Graaf et al., 1998; Hollenberg et al., 1993; Kolm and Sive, 1995; Mattioni et al., 1994). To block protein synthesis, embryos were injected with hGR mRNAs at the 16-cell stage and at stage 8–8.5 the vitelline membranes were manually removed and the embryos incubated in cycloheximide (Chx, 10 μg/ml; Kurth et al., 2005). Half of this dose of Chx is reported to block >95% of protein synthesis, as detected by either scintillation counting of [35S]methionine incorporation or protein gel electrophoresis (Cho et al., 1991). After 40 minutes the Chx medium was supplemented with Dex (10 βM) and embryos allowed to develop until siblings reached stage 12.
Whole Embryo In Situ Hybridization.
Embryos were processed for ISH as previously described (Sive et al., 2000). Anti-sense Dig-labeled RNA probes were synthesized as previously described (Yan et al., 2009). The expression patterns of Foxd4, Sox11, Gmnn, and Zic2 were compared on the experimental and control sides of embryos derived from at least three different clutches of eggs from different sets of adult parents. The frequencies at which embryos showed ectopic expression of early neural genes in the ventral epidermis were compared to controls using the Chi-squared test (p < 0.05).
Animals.
Fertilized eggs were obtained from adult frogs under in accordance with national and NIH guidelines and via protocols approved by the GWU IACUC.
ACKNOWLEDGMENTS
The authors thank Ken Cho, Igor Dawid, Eddy De Robertis, Kris Kroll, Tim Grammar, Laurent Kodjabachian, Ariel Ruiz i Altaba, Tom Sargent, and Andrey Zaraisky for providing many of the plasmids used in this study. They thank Himani Datta Majumdar for constructing the hGR-MT-Twn plasmid and Dr. Thomas Maynard, Director of the GW Biomarker Analysis Core, for bioinformatics assistance. Dr. Klein’s efforts were supported by the National Science Foundation while working at the Foundation.
Contract grant sponsor:
NSF grant, Contract grant number: MCB-1121711; Contract grant sponsor: BSF grant, Contract grant number: 2013422
Abbreviations:
- ISH
in situ hybridization
- NE
neural ectodermal
LITERATURE CITED
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. 2000. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development 127:1173–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault KT, Xu RH, Kung HF, Jamrich M. 1997. The homeobox gene PV.1 mediates specification of the prospective neural ectoderm in Xenopus embryos. Dev Biol 192:162–171. [DOI] [PubMed] [Google Scholar]
- Bae S, Reid CD, Kessler DS. 2011. Siamois and twin are redundant and essential in formation of the Spemann organizer. Dev Biol 352:367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DV, Huang S, Moody SA. 1994. The cleavage stage origin of Spemann’s organizer: Analysis of the movements of blastomere clones before and during gastrulation in Xenopus. Development 120:1179–1189. [DOI] [PubMed] [Google Scholar]
- Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. 2006. The establishment of neuronal properties is controlled by sox4 and sox11. Genes Dev 20:3475–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz IL, Cho KW 1995. Anterior neurectoderm is progressively induced during gastrulation: The role of the Xenopus homeobox gene orthodenticle. Development 121:993–1004. [DOI] [PubMed] [Google Scholar]
- Brewster R, Lee J, Ruiz i Altaba A. 1998. Gli/zic factors pattern the neural plate by defining domains of cell differentiation. Nature 393:579–583. [DOI] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. 1996. The homeobox gene siamois is a target of the wnt dorsalization pathway and triggers organizer activity in the absence of mesoderm. Development 122:3055–3065. [DOI] [PubMed] [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM. 1991. Molecular nature of Spemann’s organizer: The role of the Xenopus homeobox gene goosecoid. Cell 67:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart C, Owens NDL, Bhaw-Rosun L, Cooper B, De Domenico E, Patrushev I, Sesay AK, Smith JN, Smith JC, Gilchrist MJ. 2014. High-resolution analysis of gene activity during the Xenopus mid-blastula transition. Development 141:1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crease DJ, Dyson S, Gurdon JB. 1998. Cooperation between the activin and wnt pathways in the spatial control of organizer gene expression. PNAS USA 95: 4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LTH, Wong L, Tropepe V 2012. Zfhx1b induces a definitive neural stem cell fate in mouse embryonic stem cells. Stem Cells Development 21:2838–2851. [DOI] [PubMed] [Google Scholar]
- Darras S, Marikawa Y, Elinson RP, Lemaire P 1997. Animal and vegetal pole cells of early Xenopus embryos respond differently to maternal dorsal determinants: Implications for the patterning of the organizer. Development 124:4275–4286. [DOI] [PubMed] [Google Scholar]
- de Graaf M, Zivkovic D, Joore J. 1998. Hormone-inducible expression of secreted factors in zebrafish embryos. Dev Growth Differ 40:577–582. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. 2006. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev 7:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. 2004. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20:285–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Hausen P, Steinbeisser H. 1998. Pre-MBT patterning of early gene regulation in Xenopus: The role of the cortical rotation and mesoderm induction. Mech Dev 70:15–24. [DOI] [PubMed] [Google Scholar]
- Engleka MJ, Kessler DS. 2001. Siamois cooperates with TGFβ signals to induce the complete function of the Spemann-Mangold organizer. Int J Dev Biol 45:241–250. [PubMed] [Google Scholar]
- Fan MJ, Sokol SY. 1997. A role for siamois in Spemann organizer formation. Development 124:2581–2589. [DOI] [PubMed] [Google Scholar]
- Fetka I, Doederlein G, Bouwmeester T. 2000. Neuroectodermal specification and regionalization of the Spemann organizer in Xenopus. Mech Dev 93:49–58. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. 2006. fgf8 splice-forms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development 133: 1703–1714. [DOI] [PubMed] [Google Scholar]
- Gont LK, Fainsod A, Kim SH, De Robertis EM. 1996. Overexpression of the homeobox gene Xnot-2 leads to notochord formation in Xenopus. Dev Biol 174: 174–178. [DOI] [PubMed] [Google Scholar]
- Henningfeld KA, Rastegar S, Adler G, Knochel W. 2000. smad1 and smad4 are components of the bone morphogenetic protein-4 (BMP-4)-induced transcription complex of the Xvent-2B promoter. J Biol Chem 275:21827–21835. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Cheng PF, Weintraub H. 1993. Use of a conditional MyoD transcription factor in studies of MyoD trans-activation and muscle determination. PNAS USA 90:8028–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppler S, Moon RT. 1998. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev 71:119–129. [DOI] [PubMed] [Google Scholar]
- Houston DW, Wiley C. 2005. Maternal Xenopus zic2 negatively regulates Nodal-related gene expression during antero-posteror patterning. Development 132:4845–4855. [DOI] [PubMed] [Google Scholar]
- Hyodo-Miura J, Urushiyama S, Nagai S, Nishita M, Ueno N, Shibuya H. 2002. Involvement of NLK and sox11 in neural induction in Xenopus development. Genes Cells 7:487–496. [DOI] [PubMed] [Google Scholar]
- Imai Y, Gates MA, Melby AE, Kimelman D, Schier AF, Talbot WS. 2001. The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development 128:2407–2420. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Matsumura N, Hanafusa H, Matsumoto K, De Robertis EM, Kuroda H. 2008. Expression of siamois and twin in the blastula chordin/noggin signaling center is required for brain formation in Xenopus laevis embryos. Mech Dev 125:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Sokol SY 2014. Early Development of Epidermis and Neural Tissue. In: Moody SA, editor. Principles of Developmental Genetics”, 2nd ed. NY: Elsevier. pp 189–201. [Google Scholar]
- Kessler DS. 1997. Siamois is required for formation of Spemann’s organizer. PNAS USA 94:13017–13022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. 2005. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell 8:401–411. [DOI] [PubMed] [Google Scholar]
- Kodjabachian L, Lemaire P. 2001. Siamois functions in the early blastula to induce Spemann’s organizer. Mech Dev 108:71–79. [DOI] [PubMed] [Google Scholar]
- Kolm PJ, Sive HL. 1995. Efficient hormone-inducible protein function in Xenopus laevis. Dev Biol 171:267–272. [DOI] [PubMed] [Google Scholar]
- Kozmikova I, Candiani S, Fabian P, Gurska D, Kozmik Z. 2013. Essential role of bmp signaling and its positive feedback loop in the early cell fate evolution of chordates. Dev Biol 382:538–554. [DOI] [PubMed] [Google Scholar]
- Kroll KL, Salic AN, Evans LM, Kirschner MW. 1998. Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development 125:3247–3258. [DOI] [PubMed] [Google Scholar]
- Kroll KL. 2007. Geminin in embryonic development: Coordinating transcription and the cell cycle during differentiation. Front Biosci 12:1395–1409. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, Robertis DEM. 2004. Neural induction in Xenopus: Requirement for ectodermal and endomesodermal signals via chordin, noggin, beta-catenin, and cerberus. PLoS Biol 2:0623–0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth T, Meissner S, Schackel S, Steinbeisser H. 2005. Establishment of mesodermal gene expression patterns in early Xenopus embryos: The role of repression. Dev Dyn 233:418–429. [DOI] [PubMed] [Google Scholar]
- Laurent MN, Blitz IL, Hashimoto C, Rothbacher U, Cho KW 1997. The Xenopus homeobox gene twin mediates wnt induction of goosecoid in establishment of Spemann’s organizer. Development 124:4905–4916. [DOI] [PubMed] [Google Scholar]
- Lee HK, Lee HS, Moody SA. 2014. Neural transcription factors: From embryos to neural stem cells. Mol Cells 37:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. 1995. Expression cloning of siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 885–894. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Brivanlou AH. 2007. Proposal of a model of mammalian neural induction. Dev Biol 308:247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Liu C, Beichele S, Zhu Q, Song L, Lanner F, Jing N, Rossant J. 2013. Location of transient ectodermal progenitor potential in mouse development. Development 140:4533–4543. [DOI] [PubMed] [Google Scholar]
- Mattioni T, Louvion JF, Picard D. 1994. Regulation of protein activities by fusion to steroid binding domains. Meth Cell Biol 43:335–352. [DOI] [PubMed] [Google Scholar]
- Medina A, Wendler SR, Steinbesser H. 1997. Cortical rotation is required for the correct spatial expression of nr3, sia and gsc in Xenopus embryos. Int J Dev Biol 41:741–745. [PubMed] [Google Scholar]
- Moody SA. 1987. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev Biol 119:560–578. [DOI] [PubMed] [Google Scholar]
- Moody SA. 1999. Cell lineage analysis in Xenopus embryos. In: Tuan RS, Lo CW, editors. Methods in Molecular Biology, Vol. 135. Developmental Biology Protocols. Humana Press Inc.: Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Moody SA. 2000. Testing the cell fate commitment of single blastomeres in Xenopus laevis. In: Richter J, editor. Advances in Molecular Biology, Oxford University Press. pp. 355–381. [Google Scholar]
- Moody SA, Klein SL, Karpinski BA, Maynard TM, LaMantia AS. 2013. On becoming neural: What the embryo can tell us about differentiating neural stem cells. Amer J Stem Cells 2:74–94. [PMC free article] [PubMed] [Google Scholar]
- Mulligan KA, Cheyette BN. 2012. Wnt signaling in vertebrate neural development and function. J Neuroimmune Pharmacol 7:774–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. 1994. Normal table of Xenopus laevis (Daudin). North-Holland, Amsterdam. [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KWY. 2000. Interaction between wnt and TGF-β signaling pathways during formation of Spemann’s organizer. Nature 403:781–785. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Gawantka V, Dosch R, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. 1996. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development 122:3045–3053. [DOI] [PubMed] [Google Scholar]
- Onichtchoul D, Glinka A, Niehrs C. 1998. Requirement for Xvent-1 and Xvent-2 gene function in dorsoventral patterning of Xenopus mesoderm. Development 125:1447–1456. [DOI] [PubMed] [Google Scholar]
- Pera EM, Acosta H, Gouignard N, Climent M, Arregi I. 2014. Active signals, gradient formation and regional specificity in neural induction. Exp Cell Res 321:25–31. [DOI] [PubMed] [Google Scholar]
- Pinho S, Simonsson PR, Trevers KE, Stower MJ, Sherlock WT, Khan M, Streit A, Sheng G, Stern CD. 2011. Distinct steps of neural induction revealed by asterix, obelix and TrkC, genes induced by different signals from the organizer. PLoS One 6:e19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel MC, Lekven AC. 2004. Repression of the vertebrate organizer by wnt8 is mediated by vent and vox. Development 131:3991–4000. [DOI] [PubMed] [Google Scholar]
- Reid CD, Zhang Y, Sheets MD, Kessler DS. 2012. Transcriptional integration of wnt and nodal pathways in establishment of the Spemann organizer. Dev Biol 368:231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. 2005. Regulation of ADMP and bmp2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123:1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C, Archer TC, Cunningham DD, Grammer TC, Casey SEM. 2008. sox3 expression is maintained by FGF signaling and restricted to the neural plate by vent proteins in the Xenopus embryo. Dev Biol 313:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Ferzli GS, Casey ES. 2011. The response of early neural genes to FGF signaling and inhibition of BMP indicate the absence of a conserved neural induction module. BMC Dev Biol 11:74– doi: 10.1186/1471-213X-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Moody SA, Casey ES. 2009. Neural induction and factors that stabilize a neural fate. Birth Defects Res C: Embryo Today 87:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A, Jessell TM. 1992. Pintallavis, a gene expressed in the organizer and midline cells of frog embryos: Involvement in the development of the neural axis. Development 116:81–93. [DOI] [PubMed] [Google Scholar]
- Sasai Y 1998. Identifying the missing links: Genes that connect neural induction and primary neurogenesis in vertebrate embryos. Neuron 21:455–458. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. 1996. Beta-catenin translocation into nuclei demarcates the dorsalizing center in frog and fish embryos. Mech Dev 57:191–198. [DOI] [PubMed] [Google Scholar]
- Seo S, Herr A, Lim JW Richardson GA, Kroll KL. 2005. Geminin regulates neuronal differentiation by antagonizing brg1 activity. Genes Dev 19: 1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Kroll KL. 2006. Geminin’s double life: Chromatin connections that regulate transcription at the transition from proliferation to differentiation. Cell Cycle 5:374–379. [DOI] [PubMed] [Google Scholar]
- Sharon N, Mor I, Zahavi E, Benvenisty N. 2012. DUXO, a novel double homeobox transcription factor, is a regulator of the gastrula organizer in human embryonic stem cells. Stem Cell Res 9:261–269. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. 2000. Early Development of Xenopus laevis. A Laboratory Manual. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY. [Google Scholar]
- Snider L, Tapscott SJ. 2005. XIC is required for siamois activity and dorsoanterior development. Mol Cell Biol 25:5061–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sölter M, Köster M, Holleman T, Brey A, Pieler T, Knöchel W 1999. Characterization of a subfamily of related winged helix genes, XFD-12/12'/12• (XFLIP), during Xenopus embryogenesis. Mech Dev 89:161–165. [DOI] [PubMed] [Google Scholar]
- Stern CD. 2006. Neural induction: 10 years on since the ‘default model’. Curr Opin Cell Biol 18:692–697. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. 2000. Initiation of neural induction by FGF signaling before gastrulation. Nature 406:74–78. [DOI] [PubMed] [Google Scholar]
- Suduo N, Yamamoto S, Ogino H, Taira M. 2012. Dynamic in vivo binding of transcription factors to cis-regulatory modules of cer and gsc in the step-wise formation of the Spemann-Mangold organizer. Development 139:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SA, Akers L, Moody SA. 2001. foxD5a, a Xenopus winged helix gene, maintains an immature neural ectoderm via transcriptional repression that is dependent on the C-terminal domain. Dev Biol 232: 439–457. [DOI] [PubMed] [Google Scholar]
- Taira M, Jamrich M, Good PJ, Dawid IB. 1994. The LIM domain-containing homeobox gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev 6:356–366. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Wang T, Kroll KL. 2006. Tcf- and Vent-binding sites regulate neural-specific geminin expression in the gastrula embryo. Dev Biol 289:494–506. [DOI] [PubMed] [Google Scholar]
- Uwanogho D, Rex M, Cartwright EJ, Pearl G, Healy C, Scotting PJ, Sharpe PT. 1995. Embryonic expression of the chicken sox2, sox3 and sox11 genes suggests an interactive role in neuronal development. Mech Dev 49:23–36. [DOI] [PubMed] [Google Scholar]
- Vodicka MA, Gerhart JC. 1995. Blastomere derivation and domains of gene expression in the Spemann organizer of Xenopus laevis. Development 121:3505–3518. [DOI] [PubMed] [Google Scholar]
- Wessely O, Kim JI, Geissert D, Tran U, De Robertis EM. 2004. Analysis of Spemann organizer formation in Xenopus embryos by cDNA microarrays. Dev Biol 269:552–566. [DOI] [PubMed] [Google Scholar]
- Wills AE, Choi VM, Bennett MJ, Khokha MK, Harland RM. 2010. BMP antagonists and FGF signaling contribute to different domains of the neural plate in Xenopus. Dev Biol 337:335–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witta SE, Sato SM. 1997. 2 is a potential regulator of Spemann’s organizer. Development 124:1179–1189. XlPOU [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Hikasa H, Ono H, Taira M. 2003. Molecular link in the sequential induction of the Spemann organizer: Direct activation of the cerberus gene by Xlim-1, xotx2, mix.1 and siamois, immediately downstream from nodal and wnt signaling. Dev Biol 257:190–204. [DOI] [PubMed] [Google Scholar]
- Yan B, Neilson KM, Moody SA. 2009. foxd5 plays a critical upstream role in regulating neural fate and onset of differentiation. Dev Biol 329:80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Neilson KM, Moody SA. 2010. Microarray identification of novel downstream targets of FoxD5, a critical component of the neural ectodermal transcriptional network. Dev Dyn 239:3467–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai I, Peshkin L, Jorgensen P, Kirschner MW. 2011. Mapping gene expression in two Xenopus species: Evolutionary constraints and developmental flexibility. Dev Cell 20:483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Kim JH, Kim SC, Park JB, Lee JY, Kim J. 2014. PV.1 suppresses the expression of FoxD5b during neural induction in Xenopus embryos. Mol Cells 37:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JJ, KJolby RA, Kong NR, Monica SD, Harland RM. 2014. Spalt-like 4 promotes posterior neural fates via repression of pou5f3 family members in Xenopus. Development 141:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaraisky AG, Ecochard V Kazanskaya OV Lukyanov SA, Fesenko IV Duprat AM. 1995. The homeobox-containing gene XANF-1 may control development of the Spemann organizer. Development 121:3839–3847. [DOI] [PubMed] [Google Scholar]