Yield and molar activity of [11C]CH2O-DNPHa.
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Solventb | N-Oxide | T c (°C) | Yield (%) | A m d (GBq μmol−1) | n g | |
| EOBe | EOTf | EOTf | |||||
| 1 | DMSO | TMAO | 120 | 55 ± 7.0 | 37 ± 4.3 | 90 ± 36 | 3 |
| 2 | DMSO | NMO | 120 | 57 ± 9.3 | 38 ± 6.2 | 190 ± 29 | 5 |
| 3 | DMSO | OMT | 120 | 55 ± 9.5 | 37 ± 6.2 | 158 ± 41 | 6 |
| 4 | DPSO | OMT | 150 | 57 ± 1.5 | 39 ± 0.8 | 335 ± 52 | 4 |
| 5 | DMF | TMAO | 120 | 36 | 23 | 123 | 1 |
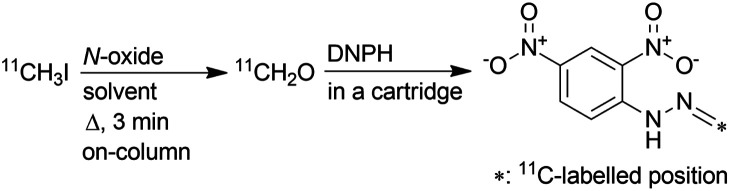
Values are presented as mean ± sd. [11C]CH2O eluted from the reaction column was introduced into a cartridge containing 2,4-dinitrophenylhydrazine (DNPH) to yield [11C]CH2O-2,4-dinitrophenylhydrazone ([11C]CH2O-DNPH).
The designated solvents (DMSO and DMF, 250 μL; DPSO, 250 mg) were mixed with MeCN (750 μL) and N-oxide (TMAO and NMO, 10 mg; OMT, 25 mg) and used to prepare the reaction column.
Temperature of reaction column.
Molar activity.
At the end of bombardment.
At the end of [11C]CH2O transfer.
Number of experiments.
