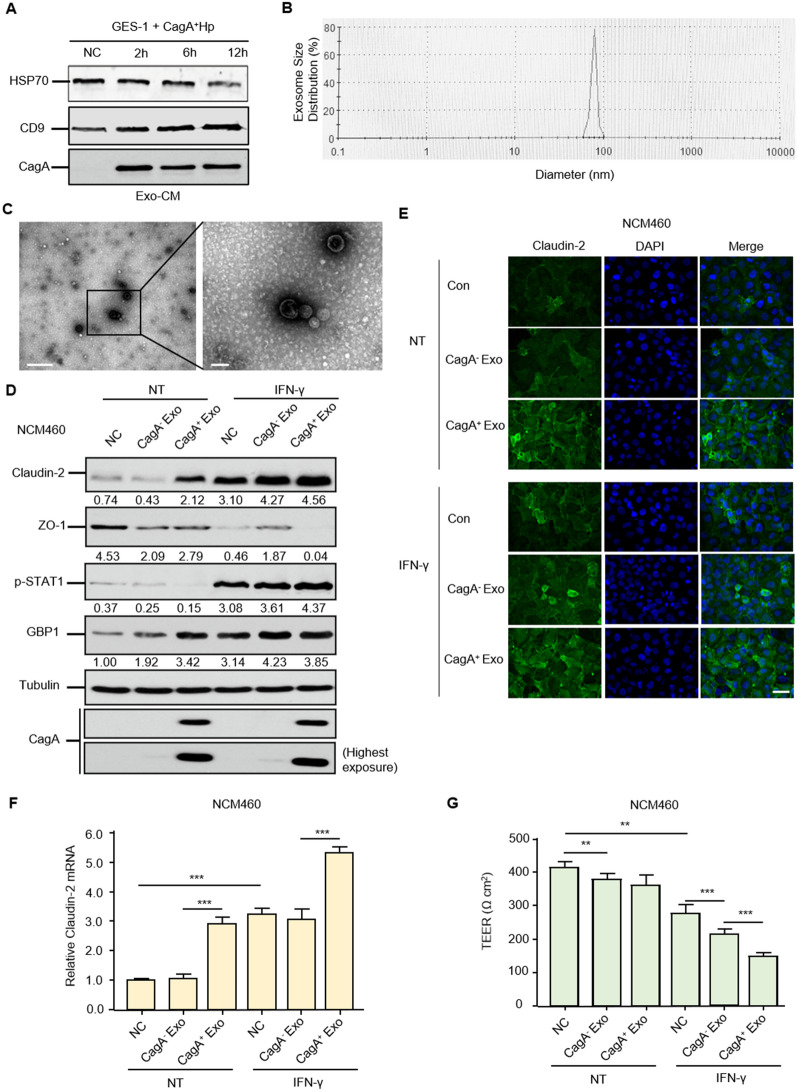
Fig. 2.
Exosomes from GES-1 human gastric epithelial cells cultured with CagA+ H. pylori significantly disrupt the intestinal mucosal barrier meanwhile and upregulate Claudin-2 in vitro. A Western blotting analysis confirmed exosomes isolated from GES-1 and CagA+ H. pylori coculture media via characteristic biomarkers HSP70 and CD9. The presence of CagA within the exosome was also shown. Exo-CM, exosome derived from conditioned medium. B, C Features of exosomes in terms of size distribution (B) and morphology (C) on transmission electron microscopy. Scale bar, 0.5 µm and 200 nm. D Western blotting verified the augmentation of Claudin-2 proteins by CagA+ exosomes, while the CagA+ exosome group partially maintained Claudin-2 protein expression under IFN-γ conditions. p-STAT1 and GBP1 were employed as positive controls to show the inflammatory status in response to CagA+ exosomes, CagA− exosomes, or IFN-γ. The p-STAT1 pathway was stringently dependent on IFN-γ, as expected, and the presence of a slight GBP1 band also showed a low degree of inflammation after the entry of CagA+ exosomes. The shrinkage of ZO-1, an acknowledged tight junction protein in both CagA+ exosomes and IFN-γ conditions, was observed as a positive control. E Cell immunostaining showed that CagA+ exosomes contributed to membrane Claudin-2 formation, whose increase was also observed in IFN-γ induced barrier function disorders. Scale bar, 20 µm. F RT–qPCR revealed the mRNA expression tendencies in different groups. G CagA+-containing exosomes accelerate the dysintegrity of the NCM460 cell monolayer under inflammatory factor IFN-γ conditions. The transepithelial electrical resistance (TEER) values of the cell monolayer were tested after CagA+ exosomes and IFN-γ 24 h. The data are expressed as the means ± SD of three independent experiments. ***p < 0.001, t test