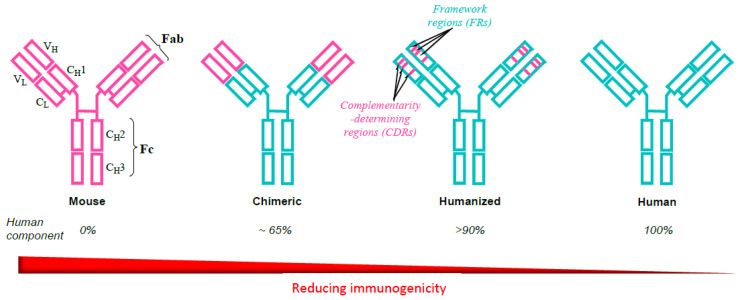
Figure 1.
Evolution of the development of therapeutic monoclonal antibodies from murine to fully human proteins to avoid unwanted immunogenicity. The iterative process proceeded stepwise through chimeric constructs incorporating mouse immunoglobulin variable regions into constant regions of human immunoglobulins and via humanized antibodies by substituting mouse complementarity determining regions (CDRs) in place of human sequences. Fully human antibodies have been developed with the application of phage display and transgenic mice technologies. Reproduced with permission from Baldo BA. Safety of biologics therapy. Monoclonal antibodies, cytokines, fusion proteins, hormones, enzymes, coagulation proteins, vaccines, botulinum toxins. Cham, Switzerland: Springer Nature; 2016 [3].