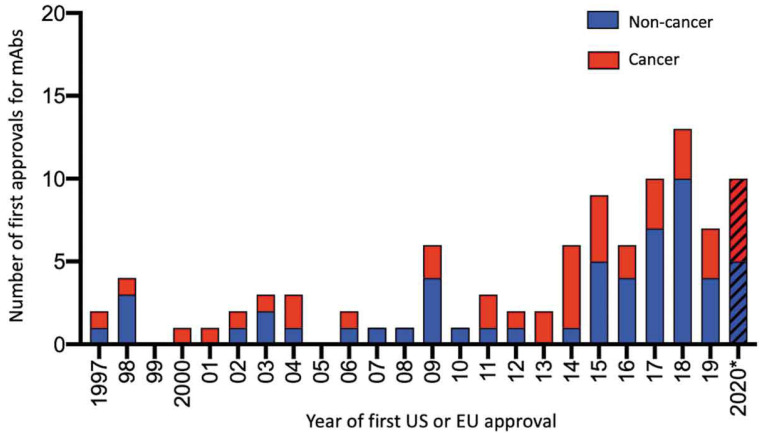
Figure 5.
Numbers of mAbs approved by the FDA and/or EMA during the 24 year period 1997–2020. Biosimilar and Fc fusion proteins are not included. Note that in 2021, 14 mAb products were approved. *: Data publicly available as of 25 November 2020. From Kaplon, H.; Reichert, J.M. Antibodies to watch in 2021. Mabs 2021, 13, e1860476, doi.org/10.1080/19420862.2020.1860476 [65], an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial License (http://creativecommons.org/licenses/by-nc/4.0) (accessed on 14 December 2021).