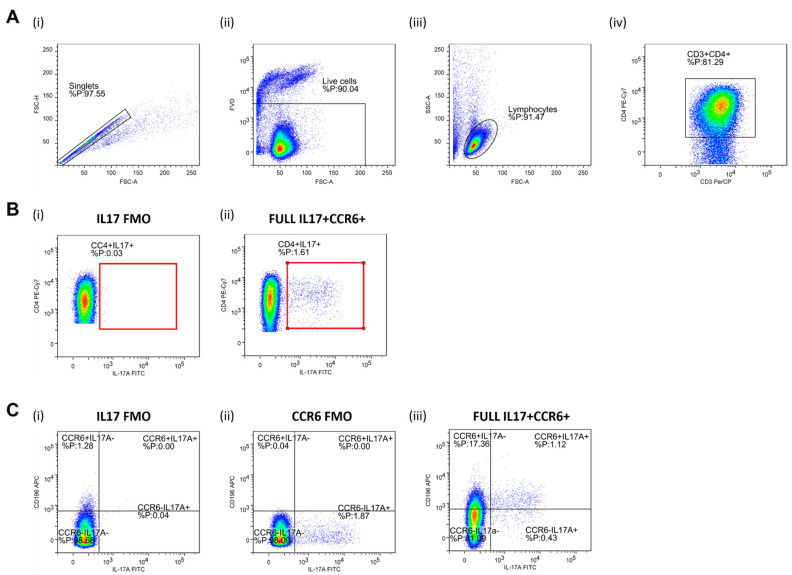
Figure 2.
Gating strategy for the assessment of peripheral blood Th17 population by flow cytometry. Panel (A) shows representative dot plots illustrating gating strategy, including exclusion of doublets using forward scatter area (FSC-A) versus forward scatter width (FSC-W) analysis (A-i), gating on live cells negative for amine-reactive fixable viability dye (A-ii), lymphocytes (A-iii), CD3+ T cells (A-iv), and CD3+CD4+ T helper cells (A-iv). T helper cells were subsequently analyzed for IL-17A and CD196/CCR6 expression. First, total IL-17-secreting T helper cells were analyzed (Panel (B)) where the gate on IL-17+ T cells were defined using florescence minus one (FMO) control for IL17 antibody (B-i). A representative sample demonstrating relative frequencies of peripheral CD4+IL-17+ T helper cells in healthy young individuals are shown at (B-ii). The population of IL-17-secreting T helper cells was further analyzed for CD196/CCR6 expression (panel (C), C-iii), hence two subpopulations were identified—CD4+CD196+IL17+ corresponding to Th17 cells and CD4+CD196-IL17+ non-Th17 cells accounting for other T helper subpopulations with the capacity to secrete IL-17. FMO controls for antibodies with specificities for IL17 and CCR6 were utilized for the setup of quadrant gates (C-i, C-ii). FlowLogic software was used for data analysis and illustration.