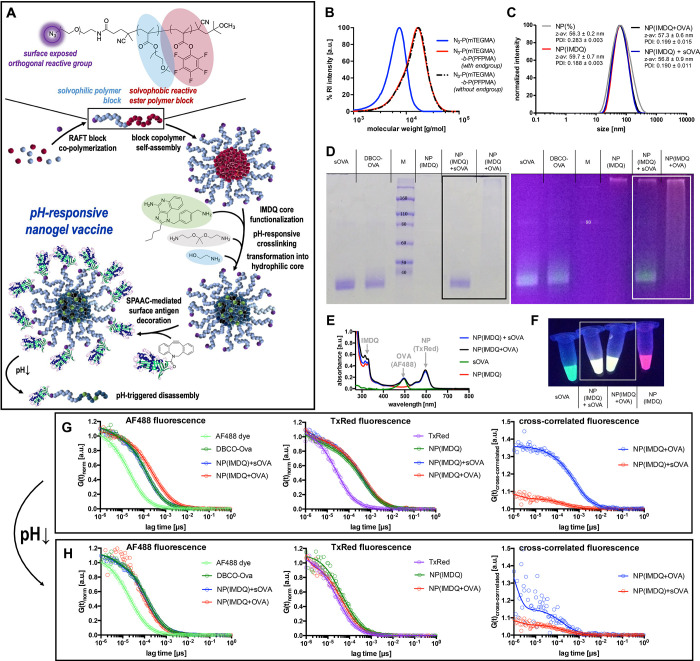
Figure 1.
Characterization of TLR7/8-agonist- and protein conjugated nanogels for precise co-delivery of adjuvant and antigen during i.v. antitumor vaccination. (A) Synthetic design concept based on double reactive precursor block copolymers that self-assemble into block copolymer micelles with amine-reactive cores and a SPAAC-reactive corona. Via aminolysis of the pentafluorophenyl esters, the cores are covalently functionalized with the TLR 7/8 agonist IMDQ and Texas Red cadaverine and then sequentially cross-linked and transformed into pH-responsive nanogels. The corona is modified via click ligation of the surface-exposed azides to DBCO-modified (and Alexa Fluor 488-labeled) OVA as model antigen. (B) SEC chromatography of the RAFT-derived reactive homo and block copolymer (before and after removal of the dithiobenzoate end group). (C) DLS intensity size distribution plots of the resulting nanogels (with and without covalent IMDQ loading), mixed or covalently modified with OVA. (D) SDS-PAGE of modified OVA (labeled with Alexa Fluor 488) mixed or covalently conjugated to IMDQ-loaded nanogels (labeled with Texas Red) (left, Coomassie staining; right, UV excitation of the fluorescent dyes (red, Texas Red-labeled nanogel; green, Alex Fluor 488-labeled OVA). (E) UV–vis spectrum of the fluorescently labeled samples and (F) corresponding image of the samples upon excitation by a UV lamp. (G) FCS correlograms derived from Alexa Fluor 488 and Texas Red fluorescence, as well as their cross-correlated correlogram indicating successful OVA conjugation to the nanogel. (H) FCS correlograms and corresponding cross-correlated correlogram upon exposure to endosomal acidic pH conditions indicating successful particle degradation.