Abstract
The oral microbiome, forming a biofilm that covers the oral structures, contains a high number of microorganisms. Biofilm formation starts from the salivary pellicle that allows bacterial adhesion–colonization–proliferation, co-aggregation and biofilm maturation in a complex microbial community. There is a constant bidirectional crosstalk between human host and its oral microbiome. The paper presents the fundamentals regarding the oral microbiome and its relationship to modulator factors, oral and systemic health. The modern studies of oral microorganisms and relationships with the host benefits are based on genomics, transcriptomics, proteomics and metabolomics. Pharmaceuticals such as antimicrobials, prebiotics, probiotics, surface active or abrasive agents and plant-derived ingredients may influence the oral microbiome. Many studies found associations between oral dysbiosis and systemic disorders, including autoimmune diseases, cardiovascular, diabetes, cancers and neurodegenerative disorders. We outline the general and individual factors influencing the host–microbial balance and the possibility to use the analysis of the oral microbiome in prevention, diagnosis and treatment in personalized medicine. Future therapies should take in account the restoration of the normal symbiotic relation with the oral microbiome.
Keywords: biofilm, metabolomics, oral diseases, systemic diseases, genomics, Oral Microbiome Database, amyloid, immune responses, autoimmune diseases
1. Introduction
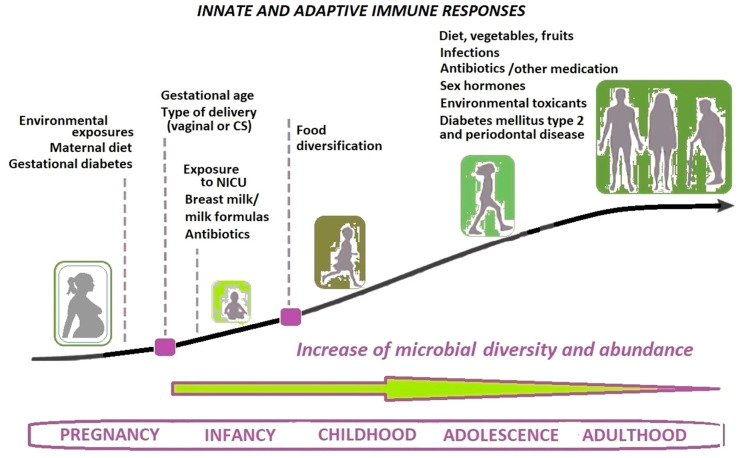
Animal bodies are host for various symbiotic microbial species, forming a complex association throughout the organism’s lifetime [1,2,3]. Bacteria were involved in animal bodies’ functions for more than 500 million years, having evolved together ever since [4]. The host factors can positively affect the microbiome, promoting balance and diversity between different types of species, resulting in a state of symbiosis and absence of pathology [5]. Moreover, co-evolution has led to interdependence: the human microbiome influences a large array of essential functions of the host, affecting a variety of physiologic, immunologic and metabolic processes, including the training and development of the host’s innate and adaptive immune system [6]. The term of microbiome, first introduced by Lederberg in 1958, signifies the ecological community of commensal, symbiotic and pathogenic microorganisms that share our body space [7]. The definition of microbiome is more complex than initially considered, encompassing, besides bacteriome, fungi (mycobiome) virus (virome) and ultrasmall organisms (candidate phyla radiation group) [8,9]. In the meantime, the host provides its microbes with an appropriate environment for their maturation and growth [5]. The human microbiome has an important body-site specificity: the oral microbiome has distinct patterns of composition and function from the microbiome of the skin, vagina or the distal gut [6]. Species or strains also vary according to the diet, age, use of antibiotics, health status, genetics, environmental exposures (to xenobiotics or microorganisms), disease state, socioeconomic status, geography and pregnancy status [6,10,11,12] (Figure 1).
Figure 1.
The main factors influencing the evolvement of human microbiome over the lifespan (adapted after [5,6,13]). NICU—neonatal intensive care unit; CS—cesarean section.
The perturbations in the size and composition of a specific microorganism’s communities have been involved in a large array of pathology: metabolic, gastrointestinal, hepatic, neurologic, autoimmune, oncologic, cardiovascular or even in psychology [6,7].
The contribution of human microbiome to disease susceptibility and to the pathogenesis and progression of systemic diseases is only beginning to be understood.
2. Modern Methods for Studying the Human Microbiome
Microbiome studying was limited until recently to the conventional culture-dependent procedures; nevertheless, the impossibility of studying all of the microbes in isolation makes the microbial investigation more difficult [14]. Nowadays, the Human Oral Microbiome Database (HOMD) [http://www.homd.org, accessed in January 2022] provides for the scientific community comprehensive information on the predominant bacterial species identifiable in the human oral cavity; this database contains the information of over 770 prokaryotic species [15,16].
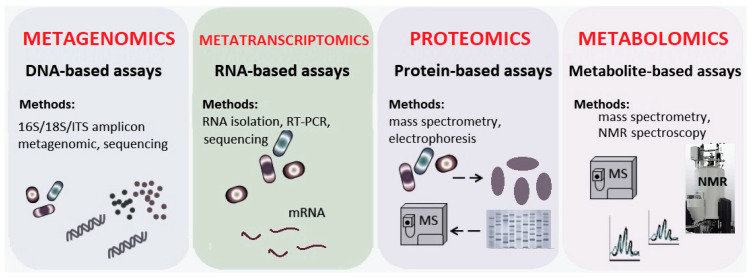
Current methods to study the oral microbiome include cultures and microscopy, gel-based techniques, polymerase chain reactions methods, DNA microarrays and Next Generation Sequencing (NGS) techniques as part of the Human Microbiome Project that enabled a better understanding of how the microbiome impacts human health and disease [16,17]. The modern approach is based on 16S/18S/ITS amplicons sequencing. The 16s rRNA gene, a highly conserved component, is the most widely used gene marker for the identification of genus and species, and thus for taxonomic significance in bacteria and archaea. Besides, 18S rRNA investigation is used in fungi for phylogenetics. In addition, the ITS (Internal Transcribed Spacer) region (including 5.8S), a universal fungi marker, is more appropriate for analyzing intra-specific genetic marker diversity in fungi. The complexity of the microbiome has resulted in genomic and metabolomics databases for which much information remains to be annotated, including chemical source (host versus microbe), chemical structures and metabolic pathways. Large-scale efforts for generation and integration of the data will be necessary to develop computational models. Modern technologies (Figure 2) readily support small molecular proteomic and metabolite surveys (targeted or untargeted) and nucleotide sequencing of RNA and DNA to assess host and microbial gene expression, taxonomic profiles and genomes [3,6,18]:
-
(a)
Metagenomics assays are answering the question “what microorganisms are there and what can they do?” and are referring to all genomes or genes encoded by a microbiota.
-
(b)
Metatranscriptomics approaches are designed for answering the question, “how do microorganisms respond and what pathways are activated?”
-
(c)
Metaproteomics approach is used to answer, “what interactions are between microorganisms and the host and what proteins are being produced?”
-
(d)
Metabolomics approach for answering the question, “what are the chemical results of their activity?” These approaches can predict microbiome–chemical interactions and their consequences.
Figure 2.
Representation of molecular approaches for modern studies host–microbiome interactions. Several aspects of the Central Dogma of Molecular Biology—illustrating the flow of genetic information from DNA to mRNA to protein—can be assessed to study host–microorganism and microorganism–microorganism interactions at the molecular level in human populations (adapted after [6,18,19]). Legend: mRNA: messenger ribonucleic acids, ITS: Internal Transcribed Spacer, RT-PCR: Reverse Transcription—Polymerase Chain Reaction, MS: mass spectrometry, NMR spectroscopy: Nuclear Magnetic Resonance spectroscopy.
3. The Microbiome of the Oral Cavity
3.1. Overview
The oral cavity hosts a large number of microorganisms, the totality of them being known as the oral microbiome, the oral flora or the oral microbiota [15]. The mouth offers a favorable habitat—appropriate humidity, temperature (37 °C) and pH (6.75–7.25) and abundant nutrients for various microbial species such as bacteria, protozoa, fungi and viruses [5]. The oral cavity, though sterile at birth, contaminates with pioneer species quickly after birth, becoming the habitat for many (more than 700) microbial species during one’s lifetime, being the second most heavily colonized part of the human body [16]. Delivery mode (vaginal or through cesarean section) [20] as well as the type of feeding (breast fed or formula fed) [21] can also influence the oral microbiome. The bidirectional, indivisible relationship between the human host and its oral microbiome, evolutionarily shaped, is a constant crosstalk. Oral flora performs physiological, metabolic, immunological, mucosal protector, nutritional and also detoxifying roles [22].
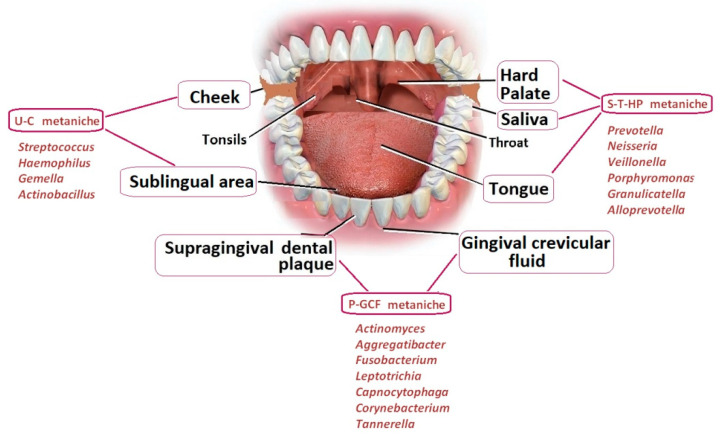
The oral microorganisms have been studied in different oral habitats: gingival sulcus, tongue, cheek, hard and soft palate, floor of the mouth, throat, saliva and teeth [15] (Figure 3). Some of the oral microorganisms have been found in all oral sites, while others showed site specificity [23,24]. A core microbiome common to all individuals as well as a variable microbiome, unique to each individual, depending on genetic and environmental factors [25], were described.
Figure 3.
The types of biological samples that have been collected during the Human Microbiome Project population: saliva, palate, tonsils, throat, buccal mucosa (cheek), tongue soft tissues, supragingival dental plaque, etc. (adapted after [15,27]; metaniches and the composition of the oral microbiota associated with anatomically diverse oral regions: U-C metaniche: sublingual-cheek region, P-GCF metaniche: supragingival dental plaque—gingival crevicular fluid region, S-T-HP metaniche: saliva–tongue–hard palate region [28].
A change in the microbiome composition or a higher number of certain microorganisms, called dysbiosis, can be associated with certain oral or systemic diseases [26].
The Human Microbiome Project (HMP) [http://www.hmpdacc.org, accessed in January 2022] was launched in 2007 by the National Institute of Health (NIH) and International Human Microbiota Consortium (IHMC) to enable large characterization of the human microbiota and investigation of their role in human health and disease [27]. The HMP studies (based upon 4788 specimens from 242 screened and phenotyped adults) have shown that oral cavity taxon may be highly personalized. The studies also revealed, despite microbial carriage variation between subjects (concerning the species and strain level), stability of metabolic pathways in healthy population [27].
The 16S rDNA profiling of the healthy oral cavity categorized the inhabitant bacteria into six broad phyla (Firmicutes, Actinobacteria, Proteobacteria, Fusobacteria, Bacteroidetes and Spirochaetes) accounting for 96% of total oral bacteria. These oral microorganisms exhibit a direct influence on human health, from host’s metabolism to immune responses [29].
In the mouth there are several niches, local microenvironments inhabited by different microbial communities, such as supra- and subgingival plaque on teeth, gingival sulcus, hard palate, tongue, sublingual area and cheek oral area [28]. In the oral cavity, most habitats were dominated by Streptococcus, but these were followed in abundance by Haemophilus in the buccal mucosa, Actinomyces in the supragingival plaque and Prevotella in the subgingival plaque. In saliva, the main species found were Streptococcus, Prevotella, Veillonella, Neisseria and Haemophilus [30]. Moreover, based upon the cluster analysis of microbial genera distribution in different oral niches, three “metaniches” with similar composition were described [28] (Figure 3).
Other non-bacterial components of the healthy oral microbiota are fungi such as Candida spp., Cladosporium, Saccharomyces, Aspergillus, Fusarium, Cryptococcus spp. and others, the latter associated with increased infection risk [30,31]. Saprophytic protozoa such as Entameba gingivalis and Trichomonas tenax, as well as Archaea have been reported as well [31]. Viruses such as herpesviruses, retroviruses and papillomaviruses are commonly found in the oral microbiota. Viruses such as Epstein–Barr virus, herpes simplex virus, HIV or hepatitis C viruses serve as reservoir for pathogenic gene functions [30,32]. SARS-CoV-2 was also found in the saliva, and inflammatory- type oral dysbiosis (including microbial species such as Veillonella and Prevotella) was associated with long COVID-19 [33].
3.2. Factors That Can Influence the Oral Microbiome
The oral microbiome characteristics are influenced by genetic and environmental factors [5,34]. The diversity and abundance of the microbiome increase from newborn period to senescence [5] (Figure 1). Variations in sex hormones may influence subgingival microbiome and periodontal disease [35]. The ecosystem’s composition and characteristics are related to several factors such as age, diet, poor oral hygiene, smoking and even dental materials of restorations, crowns, bridges and implants and also prosthetic devices, systemic diseases or medications [36,37,38,39,40,41,42,43,44]. Several host factors can negatively influence the composition of the oral microbiome to a dysbiotic state, altering the balance between the host microbiome toward a harmful relationship [5,27].
The transition in diet during human evolution led to a less diverse microbiota and a higher number of microbes associated with tooth decay and periodontal disease [36]. The diet can have habitat-specific effects on oral microbiome [45]. The refined sugar in the diet led to selection of Streptococcus mutans to outnumber other oral bacteria [46,47,48,49]. Diet is influencing periodontal diseases as well, as deficiencies in micronutrients (vitamins C, D), antioxidants, low docosahexaenoic acid and low magnesium and calcium serum values correlate with periodontal disorders [50]. Commensal bacteria have an advantage over pathogen bacteria when the host diet is not rich in fermentable carbohydrates [8].
Besides other risk factors such as age, genetics, gender, diet and oral hygiene [51], smoking was shown to determine changes in the oral microbiome [52] and also have an immunosuppressant effect [53]. Smoking results in a higher taxonomic diversity and richness in the subgingival plaque, with increased anaerobes such as Fusobacterium nucleatum. By contrast, the commensal Streptococci, Granulicatella and Actinomycetes are reduced in smokers’ saliva [30].
4. Biofilms in Oral Microbiome
4.1. Definition
Biofilms are highly-organized aggregates of microorganisms into an extracellular matrix, frequently self-produced [8,54,55]. Bacteria in biofilms have a cell-to-cell contact, a synergistic lifestyle and a set of unique characteristics different from the free-living cells [55].
The oral microbiome forms a biofilm that covers the oral structures, containing a high number of microbes influenced by the composition and surface characteristics they accumulate onto [56,57,58]. The self-produced extracellular polymeric matrix of the biofilm is mainly composed of polysaccharides, proteins, lipids and extracellular DNA [55]. Biofilm formation starts from the salivary pellicle that favors bacterial adhesion, colonization, proliferation and then co-aggregation, followed by the biofilm maturation in a complex microbial community [57]. According to the type of biofilm, bacterial gene expression changes can also occur [55,58].
The in vitro models of the plaque biofilm allowed the testing of antimicrobial or microbe modulating compounds and exploration of biochemical and metabolic interactions among different species and taxa from the oral cavity. Nevertheless, the plaques reconstituted in vitro are not similar to the oral cavity plaque with respect to the spatial structure [59]. Moreover, most studies are performed on single-species populations, whereas recent studies demonstrate the existence of co-operative behavior (for instance, a biofilm between P. aeruginosa, Pseudomonas protegens and Klebsiella pneumoniae synergistically degraded a toxin, which none of the monospecies biofilm was able to degrade) [55].
There is an increasingly known exchange of information between microbial communities in the oral microbiome, as well as with the host [60]. Streptococci and several other species are important for the spatial and temporal development of oral biofilms and its maintenance through several mechanisms, including the production of hydrogen peroxide (H2O2), also a signaling molecule [60]. H2O2 is produced by commensal flora, mainly by S. sanguis, and depends on bacterial enzymes such as pyruvate oxidase SpxB, lactate oxidase or L-aminoacid oxidase [60]. Tipping the balance to increase H2O2 production improves the oral health [60].
The self-produced extracellular polymeric matrix of the biofilm is mainly composed of polysaccharides, proteins, lipids and extracellular DNA [55]. The exposure to the toxic ingredients in smokers may relate to oral dysbiosis through immunosuppression, hypoxia or formation and colonization of biofilms with respiratory pathogens such as Haemophilus and Pseudomonas [30].
4.2. Biofilms and Curli Amyloid
Amyloids are proteins with conserved beta sheet structures, which may be produced by host cells and accumulate in tissues in various inflammatory or degenerative diseases. Amyloid fibers called curli may also be produced by bacteria containing the csg gene cluster, as a component of bacterial biofilms, and up to 40% of bacterial biofilms contain amyloids [12]. Curli fibers are produced by Enterobacteriaceae, Bacteroidetes, Proteobacteria, Firmicutes and Theromosulfobacteria [12]. Curli fibers are involved in cytokine production such as type I interferons, activate the Toll-like receptors TLR1 and TLR2 and the intracellular NLR family Pyrin Domain Containing 3 (NLRP3) inflammasome resulting in inflammation [12,61,62]. Amyloid-producing bacteria have been described in systemic lupus erythematosus, reactive arthritis, neurodegenerative diseases, colorectal cancers and other diseases, and progress in understanding their contribution to disease pathogenesis will hopefully bring about potential therapies [62].
Effective control of oral biofilms is challenging, as microorganisms in biofilms have increased drug tolerance [54]. Several strategies including antimicrobial dental materials based upon antimicrobial agent release, contact killing and combined strategies have been developed lately [54]. More bacteria accumulate on rough than on smooth surfaces of dental materials: on ceramics the biofilms are thin and highly viable, whereas on composites and glass-ionomer the cements cause surface deterioration, which enhances biofilm formation again [39]. Removable dentures favor the accumulation of microbes and biofilm growth by increasing the total surface available, their influence depending on the individual characteristics of the materials [31].
In a professional environment, antimicrobial photodynamic therapy has recently emerged as an alternative to mechanical removal of biofilms [54].
5. Oral Microbiome in Oral and Systemic Pathology
5.1. Oral Microbiome and Periodontitis
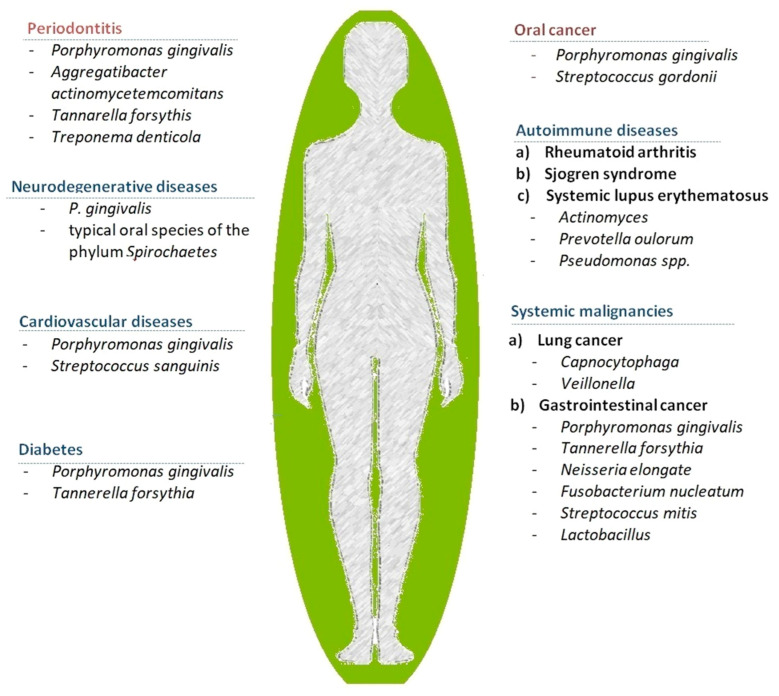
Disturbances of the symbiotic relation between the host and the oral microbiome may cause oral and systemic diseases [63,64] (Figure 4). Microbes from oral biofilm can spread in other parts of the body through the respiratory or blood systems or to the digestive tract [65]. Periodontitis (PD) is a bacterially-triggered chronic periodontal inflammation resulting in progressive, irreversible destruction of the connective periodontal attachment and alveolar bone resorption and tooth loss [66]. The common forms of PD are associated to anaerobic, Gram-negative bacteria—bacteroides such as Porphyromonas gingivalis and Prevotella intermedia and spirochetes such as Treponema denticola [66,67,68]. A higher prevalence of Actynobacillus Actinomycetemcomitans is characteristic for the localized juvenile PD [68].
Figure 4.
Types of main oral and main systemic diseases related to dysbiosis of the oral microbiome adapted after [6,30,43,44,63,64,65,69].
Oral diseases such as caries and PD are associated, besides infectious diseases such as endocarditis, with systemic diseases and have similar pathways with PD including cardiovascular, neurodegenerative, respiratory or autoimmune diseases, osteoporosis, diabetes, cancer or preterm birth [6,42,43,61,67,69,70] (Figure 4).
The improvement in periodontal status may parallel the improvement in systemic diseases and, the reverse, the treatment of these diseases could alleviate PD [67]. Early diagnostic and treatment of PD may have an important contribution in systemic disease treatment.
PD was shown to be a risk factor for atherosclerotic cardiovascular disease and thromboembolic events, by permitting the entrance of specific bacteria in the blood stream and thus initiating the inflammatory response of the host. Associations with other factors such as smoking, genetic factors and environmental pollutants can influence the disease progression [71,72].
5.2. Oral Microbiome and Diabetes Mellitus
Diabetes mellitus has a bidirectional relation: changes in the oral flora favor diabetes mellitus onset and progression, while high glycemic values alter the microbiome composition [73,74]. Periodontal therapies may improve blood glucose level and metabolic control [75,76,77,78,79,80]. Explanation for the predilection to PD in diabetes mellitus are altered neutrophil function and possibly the formation of advanced glycation endproducts (AGE) and consecutive upregulation of their receptors (RAGE), leading to increased proinflammatory cytokines production [67].
5.3. Oral Microbiome and Pulmonary Diseases
Oral bacteria may influence the respiratory pathogens colonization, and chronic obstructive pulmonary disease and pneumonia could be associated to poor oral health and PD [81,82]. Oral pathogens have also been found in broncho-alveolar fluid from patients with cystic fibrosis and improving the oral taxa composition in favor of the normal H2O2-producing Streptococcal commensals could interfere with Pseudomonas aeruginosa in this setting [60].
5.4. Oral Microbiome and Osteoporosis
Osteoporosis and PD share risk factors such as age, smoking and/or alcohol consumption, body mass index and menopause [66]. Overexpression of inflammatory cytokines may result in the vicious circle of osteoclasts activation, gingival bone resorption, increased periodontal space, bacterial proliferation and inflammation [66].
5.5. Oral Dysbiosis and Cancer
Epidemiological studies found associations between oral dysbiosis and cancers. In particular, anaerobic bacteria associated with poor oral health and PD, such as Fusobacterium nucleatum and Porphyromonas gingivalis, can play a role in oral and cervical tumorigenesis [43,83,84]. Other oral pathogens associated with carcinogenesis are aerobic bacteria such as Parvimonas, human papilloma virus in oral and cervical cancers, fungi and parasites [25,31,32,85,86]. The mechanisms of tumorigenesis related to oral microbiome, mainly in head and neck cancers, are multiple: suppression of the protective immune response, synthesis of mutagens such as aldehydes, bacterial cytotoxins promoting DNA damage or chronic inflammation [84,87].
Periodontal bacterial infections increase cancer incidence, poor survival, disease-free survival and cancer-specific survival. In this instance, P. gingivalis and Prevotella intermedia increased cancer risk, unlike Tannerela forsytihia, Treponema denticola, F. nucleatum or Aggregatibacter actinomycetemcomitans [87,88].
Some commensals in the oral cavity may be related to distant cancers: individual number variations (increase or decrease), certain organisms as predictors or changes in different indexes may function as biomarkers [87]. For instance, P. gingivalis and Fusobacterium increased in oral rinse were associated with different types of cancer in several studies [87]. The combination of Neisseria elongata and Streptococcus mitis was described as suggestive for pancreatic cancer [89]. On the contrary, some species may be protective for certain cancers, such as Neisseria in esophageal cancer, possibly by activation of carotenoid biosynthesis pathway [30,90].
5.6. Oral Dysbiosis and Autoimmune Diseases
Environmental and microbial interactions at mucosal sights could trigger autoimmunity in genetically susceptible hosts [30,91]. Gastrointestinal tract dysbiosis (microbiota composition changes, loss of beneficial with relative growth of harmful microorganisms, loss of microbial diversity) contributes to tolerance loss resulting in development of immune rheumatic diseases [92]. Oral dysbiosis could also lead to autoimmunity through multiple mechanisms including autoantigens overproduction, microbial translocation, molecular mimicry, superantigens, checkpoints dysregulation, bystander activation, TLRs dysregulation, cytokines hyperproduction, epitope spreading and autoantigens complementarity [reviewed by 61]. Genetic factors may influence the microbial–host interactions, as HLA-DR4 could enhance innate immune responses after bacterial challenge, while HLA-DQ8 could favor antigen-specific autoreactivity [91].
The development of rheumatoid arthritis (RA) may be influenced by pathogenic bacteria overgrowth, lack of immune-modulating commensal bacteria or long-lasting epigenetic changes in the synovial antigen presenting cells or in the stem cells induced by danger signals (“trained immunity”) [93]. Moreover, commensal bacteria may turn opportunistic in the oral cavity, leading to oral or systemic pathology [92,94]. Periodontal bacteria induce the neutrophil, monocyte and T and B responses with proteinases, cytokines and prostaglandins, with bone resorption similar to RA [94]. Moreover, oral microbiome profoundly influences the gut microbiome [92,95]. Lactobacillus, belonging to the Firmicutes phylum, was generally associated with anti-inflammatory activity, but its role has been regarded as controversial lately [92]. Moreover, oral bacteria DNA was found in the synovial fluid of patients with RA [96].
In rheumatoid arthritis (RA), PD is more frequent than in controls, and periodontal disease and RA share multiple risk factors, such as smoking and genetic association with HLA-DR [91]. Protein citrullination thus rendering them antigenic is involved in the disease pathogenesis, and the anticitrullinated protein antibodies (ACPA) are a disease marker. Bacteria containing the enzyme peptidylarginine deiminase (PAD) are involved in the citrullination and generation of neoantigens at mucosal sites. Prevotella intermedia, Tanerella forsythia, Treponema denticola and Porphromonas gingivalis, main bacteria involved in periodontal disease, possess PAD [97]. Moreover, anti-P. gingivalis antibodies are associated with ACPA in individuals at risk [98]. Nevertheless, the role of P. gingivalis in RA has been challenged in already-installed RA [95,99]. Moreover, Agreggatibacter actinomycetemcomitans is also a trigger for RA as it secretes leucotoxin A which contributes to neutrophil extracellular traps (NETs) generation and release of citrullinated antigens [99,100]. Microbiome switch in oral cavity might contribute to the disease pathogenesis in RA; in preclinical high-risk individuals, microbial diversity and richness was reduced compared to established RA and healthy controls [99,101]. PD is associated with RA severity and ACPA positivity [102]. Moreover, the abundance of Prevotella spp., associated with PD, along with a decrease in the normally present Streptococcus and Rothia spp., is associated with arthritis worsening and production of inflammatory mediators including interleukin-17, tumor necrosis factor-alpha (TNFα) and interferon gamma [103,104]. However, no unique oral bacterial cluster has been demonstrated to be associated with RA so far [104].
In juvenile idiopathic arthritis (JIA), some species such as Solobacterium and Mogibacterium were found enriched in saliva [105].
In Sjogren’s syndrome (SSj), a systemic autoimmune disease evolving with lymphocytic infiltration of exocrine glands, resulting hyposalivation and xerostomia are associated with increased cervical caries incidence, increased Candida, S. mutans and Lactobacillus species colonization [106,107]. Nevertheless, oral dysbiosis in SSj can occur independent of hyposalivation, with a lower oral microbial species diversity [108]. Molecular mimicry could play an important role in SSj pathogenesis, as some oral commensals such as Campnocytophaga ochraceaea along with other gut or skin bacteria contain peptides that can activate T cells reactive to Ro60, an autoantigen in SSj and systemic lupus erythematosus (SLE), in order to activate the B cells [109].
In systemic lupus erythematosus, oral ulcers are frequent and innate immunity is activated, as increased type I interferons, required for cell apoptosis and pathogen clearance, are associated with disease activity [110]. Moreover, periodontal inflammation was more frequent in SLE, associated with Fretibacterium, Prevotella nigrescens and Selenomonas spp. [111].
In systemic sclerosis, a disease evolving with cutaneous infiltration and thickening, the reduced oral aperture and the alveolar bone resorption that may occur in the disease also influence the local biomechanics and the microbial populations. The Lactobacillus spp. is reduced significantly on the tongue and in the oral cavity of systemic sclerosis patients [92]. Moreover, Lactobacilli are reduced in the diffuse form of systemic sclerosis with respect to the limited form of the disease [92].
In ankylosing spondylitis (AS), oral ulcers are more frequent than in the general population [112]. Moreover, in AS, antibodies against Porphyromonas gingivalis and Prevotella intermedia, respectively, were found in higher titer than in healthy subjects [97]. The saliva of AS patients was found to be enriched with Brucella spp. and Campylobacter concisus, in Clostridia such as Veillonellaceae, and depleted of Bacilli such as Streptococcus [113]. However other studies in AS did not find evidence of any single taxa associated with axial spondylarthritis in the subgingival plaque [114].
In Behçet’s disease, Streptococcus species have been described in the oral cavity, on mucosa and in saliva and dental plaque. S. mutans in patients with severe disease is associated with low levels of mannose-binding lectin, a host defense protein [115]. Moreover, the salivary microbiome reveals an increase in colonization with S. salivarious and S. sanguis in ulcer sites [115]. The microbial population is different at the ulceration sites with respect to other oral locations [116,117] (Table 1).
Table 1.
Oral microbioma in several autoimmune diseases.
| Disease | Microbiota Changes | References |
|---|---|---|
| Rheumatoid arthritis |
Veillonella increased in saliva of RA; Lactobacillus salivarius overrepresented in saliva, mostly in very active disease; Atopobium spp., Cryptobacillum curtum enriched in saliva and dental plaque Rothia mucilaginosa-like enriched in dental plaque and saliva; R. dentocariosa enriched in dental plaque Butyrivibrio spp., Atopobium parvulum, Prevotella spp., Solobaterium moorei, Centipeda sp., Veillonella spp., in RA Anaeroglobus geminatus correlated with ACPA and rheumatoid factor in saliva Hemophilus spp. depleted |
[95,101,102] |
| Juvenile idiopathic arthritis | Solobacterium, Mogibacterium and TM7-G1 enriched in saliva | [105] |
| Ankylosing spondylitis |
Brucella spp. and Campylobacter concisus, Clostridia such as Veillonellaceae increased in saliva Streptococcus depleted in saliva |
[97,113] |
| The saliva of AS patients enriched in Veillonella spp., Brucella spp., Campylobacter concisus and depleted in Streptococcus spp. | [113] | |
| Sjogren’s syndrome |
Lactobacillus spp., S. mutans, Candida albicans increased in supragingival plaque Fusobacterium decreases on the tongue Capnocytophaga ochracea derived microbial peptides can activate Ro60-reactive T cells |
[109,118] |
| Systemic lupus erythematosus |
Fretibacterium, Prevotella nigrescens, Selenomonas spp. are increased, associated with local release of IL-6, IL-17, IL-33 Lactobacillae, Veillonaceae and Moraxellaceae increased, while Corynebacteriaceae, Micrococcaceae, Sphingomonadaceae, Halomonadaceae and Xanthomonadaceae decreased |
[111,119] |
| Systemic scle rosis | Lactobacillus spp. are reduced significantly on the tongue, the oral mucosa, mainly in the diffuse form of disease | [92] |
| Behçet’s disease |
Streptococcus spp. increased on oral mucosa, in saliva and dental plaque, Rothia dentocariosa increased in non-ulcer sites Hemophilus parainfluenzae increased in saliva Bifidobacter dentium, Prevotella histicola, Candida albicans increased in saliva in active disease Alloprevotella rava, Campylobacter concisus, Clostridiales spp. Neisseria spp. depleted in the saliva |
[116,117,120] |
| Henoch-Schön lein purpura | Higher oral microbial diversity and richness, with dominance of Firmicutes, Proteobacteria and Bacteroidetes | [121] |
Oral microbioma may generally be influenced by the therapy of systemic autoimmune diseases. In RA, the disease-modifying drug therapy partially restored a normal oral microbiome, including increase in Prevotella spp., more abundantly found in healthy controls, and reduction in Veillonella [95]. The oral microbial signature in RA before and after methotrexate could predict the response to therapy [95]. Antibiotics may improve oral microbiome but may nevertheless induce gut dysbiosis and not improve arthritis in RA [93]. By contrast, periodontal therapy may alleviate disease activity in RA [122].
In juvenile idiopathic arthritis, no difference was found in the patients treated with biologics alone or in combination with methotrexate with respect to the microbiota [105].
In AS, sulfasalazine (an immunomodulatory drug with antibiotic properties) is effective in the peripheral form of disease [97]. In AS, the anti-TNF therapy improved the periodontal status along with the AS disease activity parameters, possibly suggesting an effect on the periodontium ligament [123].
In Behçet’s disease, besides local therapies including regular oral hygiene and topical therapies such as mouthwashes, antibiotics such as macrolides (azithromycin) also have immunomodulatory effects, decreasing the interferon responses to S. Sanguis [115]. Nevertheless, immunosuppression (cyclosporine A, azathioprine and prednisone) did not modify the modified microbioma, which was influenced by the PD therapy instead [120].
The salivary microbioma composition depends on the circadian rhythm, some genera showing significant periodicity, linking the oral microbiome with the salivary cytokine [124]. Prevotella was most significantly associated with diurnal variations of interleukins IL-1β (and to some extent to IL-6 and IL-8) [124]. However, the time of meals does not seem to influence the oral inflammatory and metabolic biomarkers [125].
Patients’ stratification and microbiome-based therapy in patients at risk or with an established autoimmune disease could be of great interest for the lifelong management of autoimmune diseases [104,113]. Variations in oral microbial species may differentiate rheumatoid arthritis from osteoarthritis, and eight bacterial biomarkers (Actinomyces, Neisseria, Neisseria subflava, Hemophilus parainfluenzae, Hemophilus, Veillonella dispar, Prevotella and Veillonella) were selected in the prediction model to help distinguish between RA and OA [126].
Oral health care, especially of microbiota, should receive attention in the daily health care of autoimmune disease patients, as the altered salivary microbiota and their metabolites may influence the disease flares and severity [93,113]. The role of probiotic supplementation in autoimmune diseases in general is controversial and requires tailored strategies which have to be proved efficient [92].
In the COVID-19 era, the widespread use of disinfectants could alter microbial diversity and load, favoring autoimmunity; moreover, quaternary ammonium compounds may impair innate immune cell function, raining concerns of a future development of autoimmune disease [127]. Parkinson’s and Alzheimer’s diseases have been reported as neurodegenerative disorders associated with peculiarities of oral dysbiosis. Animal studies (in mice) have shown that P. gingivalis infection gave brain colonization, and enzymes produced by P. gingivalis have neurotoxic effects. Moreover, an association between P. gingivalis and Alzheimer’s disease was reported [6,30]. Other microorganisms, such as Prevotella, Fusobacteria and Actinomyces, have been found in Alzheimer’s disease and in the periodontal pockets [67]. It has also been shown that typical oral species of the phylum Spirochaetes (including multiple species of the genus Treponema) often comprise amyloid plaques [6,30]. The curli-producing bacteria may also be involved, due to structural similarities between curli and human amyloids such as β-amyloid involved in Alzheimer’s disease, α-synuclein in Parkinson’s disease and serum amyloid A [128]. The molecular mechanisms of their action by oral taxa make this field an attractive area of research [6,30].
6. How to Influence the Oral Microbiome
Periodontal therapy has been shown to influence the disease activity or the biomarkers in several systemic diseases [67]. Hygiene habits influence the oral biofilm formation. Poor hygiene favors bacterial accumulation, as the salivary pellicle forms within seconds after cleaning [129]. Oral hygiene is also a key factor to prevent systemic diseases caused by the spreading of the microbes to different parts of the body [130]. Mechanical oral hygiene using dentifrice and toothbrush ensures the dental plaque removal. Mouth rinses may supplement oral hygiene and are useful in gingivitis or periodontal diseases [131]. The ingredients of dentifrices and mouth rinses influence the oral microbiome composition.
6.1. Antimicrobials
Antimicrobials, both of synthetic and natural origin, are used mainly for anti-caries benefit, being the agents with the greatest influence on the oral microbiome.
Chlorhexidine (CHX) remains the most common antimicrobial agent widely used in oral care products for its broad-spectrum and long-lasting antibacterial activity. CHX reduces the proliferation of several bacterial species linked to caries (such as Streptococcus mutans), linked to PD such as Actinomyces, Porphyromonas gingivalis, Enterobacteria, Fusobacterium nucleatum or to halitosis-related bacteria such as Porphyromonas gingivalis, Enterococcus faecalis [132,133]. The use of CHX increases favorable bacterial families such as Streptococcaceae, Carnobacteriaceae, Neisseriaceae and Flavobacteriaceae [132,134]. In the meantime, CHx use decreases Prevotellaceae, Clostridiaceae, Fusobacteriaceae, Lachnospiraceae, Campylobacteraceae, Actinomycetaceae and Corynebacteriaceae [132,134].
Cetylpiridinium chloride (CPC) 0.05% in mouth rinses exhibits antimicrobial activity against periodontal pathogens. Thus, at concentrations of 0.3–0.7%, it inhibits Actinomyces, Campylobacter, Moraxella, Veillonella, Eikenella corrodens, Porphyromonas gingivalis and Prevotellae, while at concentration of 6%, other microorganisms such as Aggregatibacter, Candida and Streptococci were inhibited [135]. Nevertheless, the long-term use of CHX and CPC with sub-lethal concentrations was associated with bacterial resistance (reported for Streptococcus mutans, Streptococcus sobrinus, Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia and Aggregatibacter actinomycetemcomitans) [136].
Sodium hypochlorite 0.05%, octenidine dihydrochloride 0.1% and povidone iodine 10% were proved to be able to reduce the vitality of periodontal pathogens such as: Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum and others [137].
6.2. Prebiotics
Prebiotics are non-viable food components that confer a benefit to the host associated with modulation of the microbiota. Arginine is an amino acid functioning as a prebiotic, which may also influence the oral ecosystem. Arginine can destabilize the oral biofilm, also decreasing the dentinal hypersensitivity and of the enamel demineralization [138,139]. Oral bacteria have an arginine deaminase system which metabolizes arginine, thus increasing the pH of the oral cavity [140]. Toothpastes with arginine (8%) significantly increased Veillonella in saliva [141]. L-arginine monohydrochloride increases Streptococcus and Veillonella and decreased Neisseria and Aggregatibacter in the oral biofilm [139].
D-tagatose, a non-cariogenic sugar abundant in the saliva of individuals with good oral health, suppresses growth of S. mutans and causes species-specific transcriptomic and metabolomic changes in S. mutans, S. gordonii and S. oralis [11].
6.3. Probiotics
The oral microbiota is influenced also by the consumption of probiotics, living microorganisms with safety profile for human ingestion which provide health benefits when they are present in specific concentrations. Lactobacillis decreases the number of S. mutans related to caries [142]. Some strains can act directly on pathogenic bacteria: Lactobacillus reuteri by producing molecules with antimicrobial activity and vitamins B12 and B6, Bifidobacterium bifidum and some species of Lactobacillus (L. johnsonii, L. crispatus, L. jensenii) are able to produce hydrogen peroxide which can act on the epithelium of other bacteria causing their death [143], L. casei produces biosurfactants which act on the preformed biofilms by dispersing them, L. acidophilus produces lipases which degrade the biofilm, while the secretory factors of L. salivarius reduces the formation of biofilm and also the pathogenicity of Candida albicans [144]. Moreover, probiotics such as Lactobacilli may attach to the mucus lining, selectively interact with host immunocompetent cells such as dendritic cells and improve the epithelial barrier function. Overall, probiotics may help control dysbiosis and alleviate local and systemic inflammation [145].
6.4. Postbiotics
Postbiotics are the microbiome-derived metabolites found in high concentration throughout the digestive system and also in the systemic circulation [34,59]. Immunomodulatory effects of postbiotics isolated from Bacillus coagulans or Bifidobacterium breve induce anti-inflammatory cell responses, and products containing L. paracasei postbiotic reduce incidence of pharyngitis [10]. Therapeutic strategies using postbiotics to modulate oral microbiome, mainly in infants, are promising directions of development [10].
6.5. Surface Active Agents
Surface active agents, such as delmopinol hydrochloride, impede the synthesis of glucan polysaccharide, a natural bioadhesive which promotes the formation of biofilm matrix and its adhesion [146]. Sodium lauryl sulfate interacts with the lipids and the proteins of the bacterial cell membrane exhibiting thus antimicrobial activity. Due to its deep penetration into oral biofilms, it was shown that it is able to inhibit plaque formation and has strong antimicrobial activity against Streptococcus mutans at concentrations ranging between 1.0 and 1.5%, at which it is usually present in dentifrices [147,148].
6.6. Abrasive Agents
Sodium bicarbonate, an abrasive agent used in dentifrices, influences the composition of oral microbiota by its pH modulating ability and its antimicrobial activity. At concentrations from 75 µM/L to 100 mM/L, it acts as bactericidal for several microorganisms from oral biofilm such as Haemophilus aphrophilus, Capnocytophaga gingivalis, Actinobacillus actinomycetemcomitans and Eikenella corrodens, and at concentrations between 52 and 65%, it decreases the level of Fusobacterium, Actinomyces sp. and S. mutans [149]. Other abrasive agents, tetrasodium and/or tetrapotassium pyrophosphate and polyphosphates, also exert antimicrobial activity. Dentifrices with tetrasodium pyrophosphate decrease Spirochetes (including Treponemae genera—T. denticola, T. vincentti, highly pathogenic at periodontal level), Proteobacteria and Fusobacteria while increasing Streptococcus genera associated with oral health [150].
6.7. Plant-Derived Ingredients
Many oral hygiene products contain extracts from plants with antimicrobial properties as an alternative to synthetic antibacterial which has several side effects and may contribute to antimicrobial resistance (Table 2).
Table 2.
Plant-derived ingredients that influence the oral microbiome.
| Plant Extracts | Plants of Essential Oils | Biological Activities on Oral Microbiome | References | |
|---|---|---|---|---|
| Camellia sinensis | Anti-inflammatory, antimicrobial, antioxidant, anti-carcinogenic and anti-allergic | [151] | ||
| Black tea (teaflavins) | Antibacterial activity against S. mutans and Porphyromonas gingivalis | [152,153] | ||
| Decreases bacteria responsible for various oral pathologies |
[154] | |||
| Melaleuca alternifolia and propolis | Eradication of Candidate albicans, Campylobacter gracilis |
[155] | ||
| Reduces Streptococcus mitis and Streptococcus sanguis |
[155] | |||
| Melaleuca alternifolia, Melissa officinalis and Lavandula angustifolia | Antibacterial activity against Staphylococcus aureus and Escherichia coli from oral biofilms | [156] | ||
| Origanum vulgare, Thymus vulgaris and Eugenia caryophyllata | Efficient against Actinomyces viscosus, Enterococcus faecalis, Streptococcus oralis, S. sanguinis, S. salivarius and S. mutans | [157] | ||
| Cymbopogon nardus | Efficient against Candida albicans and Staphylococcus aureus biofilms from prosthetic materials | [158] | ||
| Syzygium aromaticum | Good activity against Porphyromonas gingivalis | [159] | ||
| Citrus aurantium | Antimicrobial activity against S. mutans, significant decrease in several virulent genes expressed by S. mutans | [159] | ||
| Cinnamomum verum (oil from barks) |
Efficient against S. mutans | [160] | ||
| Cinamomum verum bark, Cinnamomum zeylanicum, Origanum majorana, and Melaleuca alternifolia | Efficient against Solobacterium moorei | oral bacteria responsible for halitosis | [161,162] | |
| Aloysia gratissima, Aloysia triphylla, Alpinia speciose, Artemisia capillaris, Baccharis dracunculifolia, Callitris glaucophylla, Chrysanthemum indicum, Commiphora myrrha, Coriandrum sativum, Cymbopogon citratus, Cyperus articulatus, Elyonurus muticus, Eugenia caryophyllata, Ficus deltoidea, Juniperus communis, Melaleuca alternifolia and Mentha piperita | Efficient against anaerobic bacteria Porphyromonas gingivalis and Fusobacterium nucleatum | [162] | ||
The extracts prepared from the leaves of the plant Camellia sinensis are known for their multiple biological activities: anti-inflammatory, antimicrobial, antioxidant, anti-carcinogenic and anti-allergic [151]. More specific teaflavins (Tfs), the principal active ingredients from black tea, were shown to have antibacterial activity against S. mutans [152] and Porphyromonas gingivalis [153]. Teaflavins toothpastes reduce the bacteria responsible for various oral pathologies, while not influencing the bacteria related to oral health [154].
Essential oils obtained from Melaleuca alternifolia, Melissa officinalis and Lavandula angustifolia were shown to have antibacterial activity against Staphylococcus aureus and Escherichia coli from oral biofilms [156]. Origanum vulgare, Thymus vulgaris and Eugenia caryophyllata essential oils were proven efficient against Actinomyces viscosus, Enterococcus faecalis, Streptococcus oralis, S. sanguinis, S. salivarius and S. mutans [157]. Cymbopogon nardus essential oil was shown to be efficient against Candida albicans and Staphylococcus aureus biofilms from prosthetic materials [158], and Syzygium aromaticum and several other essential oils were reported efficient against Porphyromonas gingivalis and Fusobacterium nucleatum, both related to periodontal diseases and halitosis [159,160,161,162] (Table 2).
6.8. Other Ingredients
Enzymes and proteins present in toothpastes modified the composition of the oral microflora, increasing the bacterial species associated with oral health: Prevotella melaninogenica, Neisseria sp., Granulicatella elegans and Lactobacillus gasseri, a potential probiotic with antibacterial activity against Porphyromonas gingivalis. Enzymes and protein inhibited the growth of microorganisms known to be related to periodontal diseases: Treponema, Fusobacterium, Prevotella (P. intermedia) and Eubacterium related to PD [163].
7. Oral Microbiome and Personalized Medicine
The characteristics of the oral microbiome are highly important for a complete understanding of the interactions with the host and also for the possibility of diseases prevention, diagnosis and treatment. The existing specific microbiome for each individual has an essential contribution in the onset of the disease, as disease may develop differently among different individuals [19]. The patient’s microbial profile may serve as biomarkers for evaluating the risk for disease, for early treatment, assessing response to therapy or to guide new treatments and prophylactic measures [16,29,164,165]. Research on the microbiome and its genomes in two important fields, microbiomics and metagenomics, will not only contribute to identifying the individual microbial profile but also in discovering their functions and interaction with the host [29,164]. Of interest, several studies on neonates have discussed the role that microbiota, which is altered in infants born through cesarean section, may play in early stress reactivity [25,166,167]. Knowledge on metagenome sequencing could be necessary in the future for medical practitioners for on-the-spot microbial identification and specific intervention as part of personalized medicine [25].
8. Conclusions and Perspectives
The oral microbiome is an expanding field of evaluation and research. The oral biofilm is a biological target in prevention and strategies for modulation in health and disease. A good oral hygiene and a smooth surface of dental restorations and prosthetic devices, as well as antimicrobial materials could contribute to a thinner biofilm, reducing bacterial growth and adhesion.
The progress in the extracellular matrix properties research contributes to a better understanding of the bacterial biofilm, besides the genetic and metabolic pathways [55]. Exploring the functions of the commensal oral or general microbiome and interactions with immune system, with implications in health and diseases, requires more studies. The effects of the interaction between the microbiome, virome and mycobiome add a layer of complexity in understanding their impacts on innate and adaptive immune responses. Integration of multi-omics data, including epigenomics, will help in clarifying the mechanisms that explain the high cross regulation of the oral microbiome and immune system. A successful translation of microbiome-based approaches into clinical practice needs unbiased and standardized preclinical and clinical studies. Collaboration between stomatologists, microbiologists, geneticists, pharmacists and other specialists, focused on the oral microbiome and the oral biofilm, is increasingly employed in the caring of patients with systemic diseases. Oral microbiome analysis will emerge as a new approach in prevention of systemic diseases. Future personalized therapies for oral and systemic diseases should also aim to restore the normal symbiotic relation with the oral microbiome.
Author Contributions
Conceptualization, C.B., R.V., M.L.M. and L.D.; methodology, M.L.M., C.B., L.D., S.B. and R.V.; validation, A.C. (Angela Cozma), A.F., A.C. (Adina Chiș) and L.D.; formal analysis, M.L.M., A.C. (Adina Chiș) and O.L.; data curation, A.C. (Adina Chiș) and A.C. (Angela Cozma); writing, C.B., R.V., L.D. and M.L.M.; review and editing, M.L.M., R.V., C.B. and A.C. (Adina Chiș); visualization, S.B. and O.L.; supervision, R.V., C.B., M.L.M. and L.D. All authors have read and agreed to the published version of the manuscript.
Funding
Project PDI-PFE-CDI 2021, entitled Increasing the Performance of Scientific Research, Supporting Excellence in Medical Research and Innovation, PROGRES, no. 40PFE/30.12.2021, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Margulis L. Symbiosis in Cell Evolution. 2nd ed. W.H. Freeman; New York, NY, USA: 1993. p. 452. [Google Scholar]
- 2.Gordon J., Knowlton N., Relman D.A., Rohwer F., Youle M. Superorganisms and holobionts. Microbe. 2013;8:152–153. doi: 10.1128/microbe.8.152.1. [DOI] [Google Scholar]
- 3.Bordenstein S.R., Theis K.R. Host Biology in Light of the Microbiome: Ten Principles of Holobionts and Hologenomes. PLoS Biol. 2015;13:e1002226. doi: 10.1371/journal.pbio.1002226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho I., Blaser M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012;13:260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornejo Ulloa P., van der Veen M.H., Krom B.P. Review: Modulation of the oral microbiome by the host to promote ecological balance. Odontology. 2019;107:437–448. doi: 10.1007/s10266-019-00413-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Academies of Sciences, Engineering and Medicine. Division on Earth and Life Studies, Board on Life Sciences. Board on Environmental Studies and Toxicology. Committee on Advancing Understanding of the Implications of Environmental-Chemical Interactions with the Human Microbiome . Environmental Chemicals, the Human Microbiome, and Health Risk: A Research Strategy. National Academies Press; Washington, DC, USA: 2018. [Google Scholar]
- 7.Lederberg J., Mccray A. ‘Ome sweet’ omics—A genealogical treasury of words. Scientist. 2001;15:8–10. [Google Scholar]
- 8.Bowen W.H., Burne R.A., Wu H., Koo H. Oral biofilms: Pathogens, matrix and polymicrobial interactions in microenviroments. Trends Microbiol. 2018;26:229–243. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker J.L., Batbileg B., Agnello M., Shi W., He X. Ecology of the oral microbiome: Beyond bacteria. Trends Microbiol. 2017;25:362–374. doi: 10.1016/j.tim.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zolkiewicz J., Marzec A., Ruszczynski M., Feleszko W. Postbiotics—A Step Beyond Pre- and Probiotics. Nutrients. 2020;12:2189. doi: 10.3390/nu12082189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayumi S., Kuboiwa M., Sakanaka A., Hashino E., Ishikawa A., Ijima Y., Amano A. Potential of prebiotic D-tagatose for prevention of oral disease. Front. Cell. Infect. Microbiol. 2021;11:767944. doi: 10.3389/fcimb.2021.767944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallo P.M., Rapsinski G.J., Wilson R.P., Oppong G.O., Sriram U., Goulian M., Buttaro B., Caricchio R., Gallucci S., Tükel Ç. Amyloid-DNA Composites of Bacterial Biofilms Stimulate Autoimmunity. Immunity. 2015;42:1171–1184. doi: 10.1016/j.immuni.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dethlefsen L., Relman D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. USA. 2011;108((Suppl. 1)):4554–4561. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller M., Ramos J.L. Microbial goods from single cells and metagenomes. Curr. Opin. Microbiol. 2008;11:195–197. doi: 10.1016/j.mib.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Dewhirst F.E., Chen T., Izard J., Paster B.J., Tanner A.C., Yu W.H., Lakshmanan A., Wade W.G. The human oral microbiome. J. Bacteriol. 2010;192:5002–5017. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnan K., Chen T., Paster B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017;23:276–286. doi: 10.1111/odi.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deo P.N., Deshmukh R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Path. 2019;23:122–128. doi: 10.4103/jomfp.JOMFP_304_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilhan Z.E. Ph.D. Thesis. Arizona State University; Tempe, AZ, USA: 2016. Microbiome after Bariatric Surgery and Microbial Insights into Surgical Weight Loss. [Google Scholar]
- 19.Beckonert O., Keun H.C., Ebbels T.M., Bundy J., Holmes E., Lindon J.C., Nicholson J.K. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat. Prot. 2007;2:2692–2703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 20.Holgerson P.L., Harnevik L., Hernell O., Tanner A.C., Johansson I. Mode of birth delivery affects oral microbiota in infants. J. Dent. Res. 2011;90:1183–1188. doi: 10.1177/0022034511418973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holgerson P.L., Vestman N.R., Claesson R., Ohman C., Domellöf M., Tanner A.C., Hernell O., Johansson I. Oral microbial profile discriminates breast-fed from formula-fed infants. J. Pediatr. Gastroenterol. Nutr. 2013;56:127–136. doi: 10.1097/MPG.0b013e31826f2bc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilian M., Chapple I.L., Hannig M., Marsh P.D., Meuric V., Pedersen A.M., Tonetti M.S., Wade W.G., Zaura E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016;221:657–666. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 23.Mager D.L., Ximenez-Fyvie L.A., Haffajee A.D., Socransky S.S. Distribution of selected bacterial species on intraoral surfaces. J. Clin. Periodontol. 2003;30:644–654. doi: 10.1034/j.1600-051X.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 24.Kazor C.E., Mitchell P.M., Lee A.M., Stokes L.N., Loesche W.J., Dewhirst F.E., Paster B.J. Diversity of bacterial populations on the tongue dorsa of patients with halitosis and healthy patients. J. Clin. Microbiol. 2003;41:558–563. doi: 10.1128/JCM.41.2.558-563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarco M.F., Vess T.J., Ginsburg G.S. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2012;18:109–120. doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita Y., Takeshita T. The oral microbiome and human health. J. Oral Sci. 2017;59:201–206. doi: 10.2334/josnusd.16-0856. [DOI] [PubMed] [Google Scholar]
- 27.Gao L., Xu T., Huang G., Jiang S., Gu Y., Chen F. Oral microbiomes: More and more importance in oral cavity and whole body. Prot. Cell. 2018;9:488–500. doi: 10.1007/s13238-018-0548-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seidel C.L., Gerlach R.G., Wiedemann P., Weider M., Rodrian G., Hader M., Frey B., Gaipl U.S., Bozec A., Cieplik F., et al. Defining Metaniches in the Oral Cavity According to Their Microbial Composition and Cytokine Profile. Int. J. Mol. Sci. 2020;21:8218. doi: 10.3390/ijms21218218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma D., Garg P.K., Dubey A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018;200:525–540. doi: 10.1007/s00203-018-1505-3. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y.H., Chung S.W., Auh Q.S., Hong S.J., Lee Y.A., Jung J., Lee G.J., Park H.J., Shin S.I., Hong J.Y. Progress in oral microbiome related to oral and systemic diseases: An update. Diagnostics. 2021;11:1283. doi: 10.3390/diagnostics11071283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radford D.R., Challacombe S.J., Walter J.D. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit. Rev. Oral Biol. Med. 1999;10:99–116. doi: 10.1177/10454411990100010501. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee S., Tian T., Wei Z., Peck K.N., Shih N., Chalian A.A., O’Malley B.W., Weinstein G.S., Feldman M.D., Alwine J., et al. Microbial Signatures Associated with Oropharyngeal and Oral Squamous Cell Carcinomas. Sci. Rep. 2017;7:4036. doi: 10.1038/s41598-017-03466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haran J.P., Bradley E., Zeamer A.L., Cincotta L., Salive M.-C., Dutta P., Mutaawe S., Anya O., Meza-Segura M., Moormann A.M., et al. Inflammation-type dysbiosis of the oral microbiome associates with the duration of COVID-19 symptoms and long COVID. JCI Insight. 2021;6:e152346. doi: 10.1172/jci.insight.152346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng D., Liwinski T., Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mascarenhas P., Gapski R., Al-Shammari K., Wang H.L. Influence of sex hormones on the periodontium. J. Clin. Periodontol. 2003;30:671–681. doi: 10.1034/j.1600-051X.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 36.David R. Diet and oral microbiota go hand in hand. Nat. Rev. Microbiol. 2013;11:223. doi: 10.1038/nrmicro3000. [DOI] [Google Scholar]
- 37.Marsh P.D., Head D.A., Devine D.A. Prospects of oral disease control in the future—An opinion. J. Oral Microbiol. 2014;6:26176. doi: 10.3402/jom.v6.26176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J., Peters B.A., Dominianni C., Zhang Y., Pei Z., Yang L., Ma Y., Purdue M.P., Jacobs E.J., Gapstur S.M., et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016;10:2435–2446. doi: 10.1038/ismej.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busscher H.J., Rinastiti M., Siswomihardjo W., van der Mei H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010;89:657–665. doi: 10.1177/0022034510368644. [DOI] [PubMed] [Google Scholar]
- 40.Øilo M., Bakken V. Biofilm and Dental Biomaterials. Materials. 2015;8:2887–2900. doi: 10.3390/ma8062887. [DOI] [Google Scholar]
- 41.Spahr A., Klein E., Khuseyinova N., Boeckh C., Muche R., Kunze M., Rothenbacher D., Pezeshki G., Hoffmeister A., Koenig W. Periodontal infections and coronary heart disease: Role of periodontal bacteria and importance of total pathogen burden in the Coronary Event and Periodontal Disease (CORODONT) study. Arch. Int. Med. 2006;166:554–559. doi: 10.1001/archinte.166.5.554. [DOI] [PubMed] [Google Scholar]
- 42.Gomes-Filho I.S., Passos J.S., da Cruz S.S. Respiratory disease and the role of oral bacteria. J. Oral Microbiol. 2010;2:5811. doi: 10.3402/jom.v2i0.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teles F., Alawi F., Castilho R.M., Wang Y. Association or Causation? Exploring the Oral Microbiome and Cancer Links. J. Dent. Res. 2020;99:1411–1424. doi: 10.1177/0022034520945242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nichols R.G., Peters J.M., Patterson A.D. Interplay between the Host, the Human Microbiome, and Drug Metabolism. Hum. Genom. 2019;13:27. doi: 10.1186/s40246-019-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kato I., Vasquez A., Moyerbrailean G., Land S., Djuric Z., Sun J., Lin H.S., Ram J.L. Nutritional Correlates of Human Oral Microbiome. J. Am. Coll. Nutr. 2017;36:88–98. doi: 10.1080/07315724.2016.1185386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cornejo O.E., Lefébure T., Bitar P.D., Lang P., Richards V.P., Eilertson K., Do T., Beighton D., Zeng L., Ahn S.J., et al. Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol. Biol. Evol. 2013;30:881–893. doi: 10.1093/molbev/mss278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh P. Marsh and Martin’s Oral Microbiology. 6th ed. Elsevier; Edinburgh, UK: 2016. [Google Scholar]
- 48.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Dis. Primers. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi N., Nyvad B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 50.Van der Velden U., Kuzmanova D., Chapple I.L. Micronutritional approaches to periodontal therapy. J. Clin. Periodontol. 2011;38((Suppl. 11)):142–158. doi: 10.1111/j.1600-051X.2010.01663.x. [DOI] [PubMed] [Google Scholar]
- 51.Timmerman M.F., van der Weijden G.A. Risck factors for periodontitis. Int. J. Dent. Hyg. 2005;4:2–7. doi: 10.1111/j.1601-5037.2006.00168.x. [DOI] [PubMed] [Google Scholar]
- 52.Tomar S.L., Asma S. Somoking-Attributable Periodontitis in the United States: Findings from NHANES III. J. Periodontol. 2000;7:743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 53.Tymkiw K.D., Thunell D.H., Johnson G.K., Joly S., Burnell K.K., Cavanaugh J.E., Brogden K.A., Guthmiller J.M. Influence of smoking on gingival crevicular fluid cytokines in severe chronic periodontitis. J. Clin. Periodontol. 2011;38:219–228. doi: 10.1111/j.1600-051X.2010.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiao Y., Tay F.R., Niu L., Chen J. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019;11:28. doi: 10.1038/s41368-019-0062-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flemming H.C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 56.Scotti E., Boué S., Sasso G.L., Zanetti F., Belcastro V., Poussin C., Sierro N., Battey J., Gimalac A., Ivanov N.V., et al. Exploring the microbiome in health and disease: Implications for toxicology. Toxicol. Res. Appl. 2017;1:239784731774188. doi: 10.1177/2397847317741884. [DOI] [Google Scholar]
- 57.Teughels W., Van Assche N., Sliepen I., Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006;17((Suppl. 2)):68–81. doi: 10.1111/j.1600-0501.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 58.Lamont R.J., Bryers J.D. Biofilm-induced gene expression and gene transfer. Methods Enzym. 2001;336:84–94. doi: 10.1016/s0076-6879(01)36581-3. [DOI] [PubMed] [Google Scholar]
- 59.Welch J.L.M., Rossetti B.J., Rieken C.W., Dewhirst F.E., Borisy G.G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA. 2016;113:E791–E800. doi: 10.1073/pnas.1522149113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Redanz S., Cheng X., Giacaman R.A., Pfeifer C.S., Merritt J., Kreth J. Live and let die: Hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 2018;33:337–352. doi: 10.1111/omi.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suárez L.J., Garzón H., Arboleda S., Rodríguez A. Oral dysbiosis and autoimmunity: From local periodontal responses to an imbalanced systemic immunity. A review. Front. Immunol. 2020;11:591255. doi: 10.3389/fimmu.2020.591255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller A.L., Pasternak J.A., Medeiros N.J., Nicastro L.C., Tursi S.A., Hansen E.G., Krochak R., Sokaribo A.S., MacKenzie K.D., Palmer M.B., et al. In vivo synthesis of bacterial amyloid curli contributes to joint inflammation during S. typhimurium infection. PLoS Pathog. 2020;16:e1008591. doi: 10.1371/journal.ppat.1008591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sampaio-Maia B., Caldas I.M., Pereira M.L., Pérez-Mongiovi D., Araujo R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016;97:171–210. doi: 10.1016/bs.aambs.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Dörfer C., Benz C., Aida J., Campard G. The relationship of oral health with general health and NCDs: A brief review. Int. Dent. J. 2017;67((Suppl. 2)):14–18. doi: 10.1111/idj.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Debelian G.J., Olsen I., Tronstad L. Systemic diseases caused by oral microorganisms. Dent. Traumatol. 1994;10:57–65. doi: 10.1111/j.1600-9657.1994.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 66.Contaldo M., Itro A., Lajolo C., Gioco G., Inchingolo F., Serpico R. Overview of osteoporosis, periodontitis and oral dysbiosis: The emerging role of oral microbiota. Appl. Sci. 2020;10:6000. doi: 10.3390/app10176000. [DOI] [Google Scholar]
- 67.Holmstrup P., Damgaard C., Olsen I., Klinge B., Flybjerg A., Nielsen C.H., Hansen P.R. Comorbidity of oeriodontal diseases: Two sides of the same coin? J. Oral Microbiol. 2017;9:1332710. doi: 10.1080/20002297.2017.1332710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loesche W.J., Grossman N.S. Periodontal disease as a specific, albeit chronic, infection: Diagnosis and treatment. Clin. Microbiol. Rev. 2001;14:727–752. doi: 10.1128/CMR.14.4.727-752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beck J.D., Offenbacher S. Systemic effects of periodontitis: Epidemiology of periodontal disease and cardiovascular disease. J. Periodontol. 2005;76:2089–2100. doi: 10.1902/jop.2005.76.11-S.2089. [DOI] [PubMed] [Google Scholar]
- 70.Matsha T.E., Prince Y., Davids S., Chikte U., Erasmus R.T., Kengne A.P., Davison G.M. Oral Microbiome Signatures in Diabetes Mellitus and Periodontal Disease. J. Dent. Res. 2020;99:658–665. doi: 10.1177/0022034520913818. [DOI] [PubMed] [Google Scholar]
- 71.Beck J., Garcia R., Heiss G., Vokonas P.S., Offenbacher S. Periodontal disease and cardiovascular disease. J. Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 72.Tonetti M.S., Van Dyke T.E., working group 1 of the joint EFP/AAP workshop Periodontitis and atherosclerotic cardiovascular disease: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013;84((Suppl. 4)):S24–S29. doi: 10.1902/jop.2013.1340019. [DOI] [PubMed] [Google Scholar]
- 73.Taylor G.W. Bidirectional interrelationships between diabetes and periodontal diseases: An epidemiologic perspective. Ann. Periodontol. 2001;6:99–112. doi: 10.1902/annals.2001.6.1.99. [DOI] [PubMed] [Google Scholar]
- 74.Mealey B.L., Oates T.W., American Academy of Periodontology Diabetes mellitus and periodontal diseases. J. Periodontol. 2006;77:1289–1303. doi: 10.1902/jop.2006.050459. [DOI] [PubMed] [Google Scholar]
- 75.Darré L., Vergnes J.N., Gourdy P., Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: A meta-analysis of interventional studies. Diabetes Metab. 2008;34:497–506. doi: 10.1016/j.diabet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 76.Madden T.E., Herriges B., Boyd L.D., Laughlin G., Chiodo G., Rosenstein D. Alterations in HbA1c following minimal or enhanced non-surgical, non-antibiotic treatment of gingivitis or mild periodontitis in type 2 diabetic patients: A pilot trial. J. Contemp. Dent. Pract. 2008;9:9–16. doi: 10.5005/jcdp-9-5-9. [DOI] [PubMed] [Google Scholar]
- 77.Lalla E., Kaplan S., Yang J., Roth G.A., Papapanou P.N., Greenberg S. Effects of periodontal therapy on serum C-reactive protein, sE-selectin, and tumor necrosis factor-alpha secretion by peripheral blood-derived macrophages in diabetes. A pilot study. J. Periodontal Res. 2007;42:274–282. doi: 10.1111/j.1600-0765.2006.00945.x. [DOI] [PubMed] [Google Scholar]
- 78.Correa F.O., Gonçalves D., Figueredo C.M., Bastos A.S., Gustafsson A., Orrico S.R. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J. Clin. Periodontol. 2010;37:53–58. doi: 10.1111/j.1600-051X.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- 79.Signorini L., Inchingolo A.D., Santacroce L., Xhajanka E., Altini V., Bordea I.R., Dipalma G., Cantore S., Inchingolo F. Efficacy of combined sea salt based oral rinse with xylitol in improving healing process and oral hygiene among diabetic population after oral surgery. J. Biol. Regul. Homeost. Agents. 2020;34:1617–1622. doi: 10.23812/20-418-L. [DOI] [PubMed] [Google Scholar]
- 80.Persson R.G. Diabetes and periodontal disease: An update for health care professionals. Diabetes Spectr. 2011;24:195–198. doi: 10.2337/diaspect.24.4.195. [DOI] [Google Scholar]
- 81.Scannapieco F.A., Mylotte J.M. Relationships between periodontal disease and bacterial pneumonia. J. Periodontol. 1996;67((Suppl. 10)):1114–1122. doi: 10.1902/jop.1996.67.10s.1114. [DOI] [PubMed] [Google Scholar]
- 82.Scannapieco F.A., Ho A.W. Potential associations between chronic respiratory disease and periodontal disease: Analysis of National Health and Nutrition Examination Survey III. J. Periodontol. 2001;72:50–56. doi: 10.1902/jop.2001.72.1.50. [DOI] [PubMed] [Google Scholar]
- 83.Sun J., Tang Q., Yu S., Xie M., Xie Y., Chen G., Chen L. Role of the oral microbiota in cancer evolution and progression. Cancer Med. 2020;9:6306–6321. doi: 10.1002/cam4.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medsaniity M., Hasnat S., Salo T., Salem A. Oral microbioma—A new frontier in the pathogenesi of management of head and neck cancer. Cancers. 2022;14:46. doi: 10.3390/cancers14010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao H., Chu M., Huang Z., Yang X., Ran S., Hu B., Zhang C., Liang J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017;7:11773. doi: 10.1038/s41598-017-11779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.He Y.F., Zhang M.Y., Feng L.D., Yin Y.H., Zhang R., Di W. Risk of cervical cancer and precancerous diseases in the oral HPV carriers. Zhonghua Fu Chan Ke Za Zhi. 2013;48:611–616. [PubMed] [Google Scholar]
- 87.Irfan M., Delgado R.Z.R., Farias-Lopez J. The oral microbiome and cancer. Front. Immunol. 2020;11:591088. doi: 10.3389/fimmu.2020.591088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao L., Zhang Q., Peng Y., Wang D., Liu Y. The incidence of periodontal Bacteria infection and incidence and prognosis of cancer. A systematic review and meta-analysis. Medicine. 2020;99:15. doi: 10.1097/MD.0000000000019698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farrell J.J., Zhang L., Zhou H., Chia D., Elashoff D., Akin D., Paster B.J., Joshipura K., Wong D.T.W. Variation of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61:582–588. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peters B.A., Wu J., Pei Z., Yang L., Purdue M.P., Freedman N.D., Jacobs E.J., Gapstur S.M., Hayes R.B., Ahn J. Oral microbiome reflects prospective risk for esophageal cancers. Cancer Res. 2017;77:6777–6787. doi: 10.1158/0008-5472.CAN-17-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Catrina A.I., Deane K.D., Scher J.U. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology. 2016;55:391–402. doi: 10.1093/rheumatology/keu469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Melchiorre D., Ceccherini M.T., Romano E., Cometi L., El-Aoufy K., Bellando-Randone S., Roccotelli A., Bruni C., Moggi-Pignone A., Carboni D., et al. Oral Lactobacillus species in systemic sclerosis. Microorganisms. 2021;9:1298. doi: 10.3390/microorganisms9061298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Berthelot J.M., Bandiaky O.N., Le Goff B., Amador G., Chaux A.G., Soueidan A., Denis F. Another look at the contribution of oral microbiota to the pathogenesis of rheumatoid arthritis: A narrative review. Microorganisms. 2021;10:59. doi: 10.3390/microorganisms10010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martu M.A., Solomon S.M., Sufaru I.G., Jelihovschi I., Martu S., Rezus E., Surdu A.E., Onea R.M., Grecu G.P., Foia L. Study on the prevalence of periodontopathogenic bacteria in serum and subgingival bacterial plaque in patients with rheumatoid arthritis. Rev. Chim. 2017;68:1946–1950. doi: 10.37358/RC.17.8.5798. [DOI] [Google Scholar]
- 95.Zhang X., Zhang D., Jia H., Feng Q., Wang D., Liang D., Wu X., Li J., Tang L., Li Y., et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015;21:895–905. doi: 10.1038/nm.3914. [DOI] [PubMed] [Google Scholar]
- 96.Moen K., Brun J.G., Valen M., Skartveit L., Eribe E.K., Olsen I., Jonsson R. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin. Exp. Rheumatol. 2006;24:656–663. [PubMed] [Google Scholar]
- 97.Ogrendik M. Periodontal pathogens are likely to be responsible for the development of ankylosing spondylitis. Curr. Rheumatol. Rev. 2015;11:47–49. doi: 10.2174/1573397111666150522094821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johansson L., Sherina N., Kharlamova N., Potempa B., Larsson B., Israelsson L., Potempa J., Rantapää-Dahlqvist S., Lundberg K. Concentration of antibodies against Porphyromonas gingivalis is increased before the onset of symptoms of rheumatoid arthritis. Arthritis Res. Ther. 2016;18:201. doi: 10.1186/s13075-016-1100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tong Y., Zheng L., Qing P., Zhao H., Li Y., Su L., Zhang Q., Zhao Y., Luo Y., Liu Y. Oral microbiota perturbations are linked to high risk for rheumatoid arthritis. Front. Cell. Infect. Microbiol. 2020;9:475. doi: 10.3389/fcimb.2019.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Konig M.F., Abusleme L., Reinholdt J., Palmer R.J., Teles R.P., Sampson K., Rosen A., Nigrovic P.A., Sokolove J., Giles J.T., et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016;8:369ra176. doi: 10.1126/scitranslmed.aaj1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scher J.U., Ubeda C., Equinda M., Khanin R., Buischi Y., Viale A., Lipuma L., Attur M., Pillinger M.H., Weissmann G., et al. Periodontal disease and the oral microbiota in new-onset rheumatoid arthritis. Arthritis Rheum. 2012;64:3083–3094. doi: 10.1002/art.34539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eriksson K., Fei G., Lundmark A., Benchimol D., Lee L., Hu Y.O.O., Kats A., Saevarsdottir S., Catrina A.I., Klinge B., et al. Periodontal health and oral microbiota in patients with rheumatoid arthritis. J. Clin. Med. 2019;8:630. doi: 10.3390/jcm8050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corrêa J.D., Fernandes G.R., Calderaro D.C., Mendonça S., Silva J.M., Albiero M.L., Cunha F.Q., Xiao E., Ferreira G.A., Teixeira A.L., et al. Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Sci. Rep. 2019;9:8379. doi: 10.1038/s41598-019-44674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Drago L., Zuccotti G.V., Romanò C.L., Goswami K., Villafañe J.H., Mattina R., Parvizi J. Oral-gut microbiota and arthritis: Is there an evidence-based axis? J. Clin. Med. 2019;8:1753. doi: 10.3390/jcm8101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Frid P., Baraniya D., Halbig J., Rypdal V., Songstad N.T., Rosèn A., Berstad J.R., Flatø B., Alakwaa F., Gil E.G., et al. Salivary oral microbiome of children with juvenile idiopathic arthritis: A norwegian cross-sectional study. Front. Cell. Infect. Microbiol. 2020;10:602239. doi: 10.3389/fcimb.2020.602239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shinozaki S., Moriyama M., Hayashida J.N., Tanaka A., Maehara T., Ieda S., Nakamura S. Close association between oral Candida species and oral mucosal disorders in patients with xerostomia. Oral Dis. 2012;18:667–672. doi: 10.1111/j.1601-0825.2012.01923.x. [DOI] [PubMed] [Google Scholar]
- 107.Zorba M., Melidou A., Patsatsi A., Ioannou E., Kolokotronis A. The possible role of oral microbiome in autoimmunity. Int. J. Womens Dermatol. 2020;6:357–364. doi: 10.1016/j.ijwd.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rusthen S., Kristoffersen A.K., Young A., Galtung H.K., Petrovski B.É., Palm Ø., Enersen M., Jensen J.L. Dysbiotic salivary microbiota in dry mouth and primary Sjögren’s syndrome patients. PLoS ONE. 2019;14:e0218319. doi: 10.1371/journal.pone.0218319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szymula A., Rosenthal J., Szczerba B.M., Bagavant H., Fu S.M., Deshmukh U.S. T cell epitope mimicry between Sjögren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Clin. Immunol. 2014;152:1–9. doi: 10.1016/j.clim.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Talotta R., Atzeni F., Ditto M.C., Gerardi M.C., Sarzi-Puttini P. The microbiome in connective tissue diseases and vasculitides: An updated narrative review. J. Immunol. Res. 2017;2017:6836498. doi: 10.1155/2017/6836498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Corrêa J.D., Calderaro D.C., Ferreira G.A., Mendonça S.M., Fernandes G.R., Xiao E., Teixeira A.L., Leys E.J., Graves D.T., Silva T.A. Subgingival microbiota dysbiosis in systemic lupus erythematosus: Association with periodontal status. Microbiome. 2017;5:34. doi: 10.1186/s40168-017-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Abbood H.M., Pathan E., Cherukara G.P. The link between ankylosing spondylitis and oral health conditions: Two nested case-control studies using data of the UK Biobank. J. Appl. Oral Sci. 2018;27:e20180207. doi: 10.1590/1678-7757-2018-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lv L., Jiang H., Yan R., Xu D., Wang K., Wang Q., Chen X., Li L. The salivary microbiota, cytokines, and metabolome in patients with ankylosing spondylitis are altered and more proinflammatory than those in healthy controls. mSystems. 2021;6:e0117320. doi: 10.1128/mSystems.01173-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bisanz J.E., Suppiah P., Thomson W.M., Milne T., Yeoh N., Nolan A., Ettinger G., Reid G., Gloor G.B., Burton J.P., et al. The oral microbiome of patients with axial spondyloarthritis compared to healthy individuals. PeerJ. 2016;4:e2095. doi: 10.7717/peerj.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mumcu G., Fortune F. Oral health and its aetiological role in Behçet’s disease. Front. Med. 2021;8:613419. doi: 10.3389/fmed.2021.613419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seoudi N., Bergmeier L.A., Drobniewski F., Paster B., Fortune F. The oral mucosal and salivary microbial community of Behçet’s syndrome and recurrent aphthous stomatitis. J. Oral Microbiol. 2015;7:27150. doi: 10.3402/jom.v7.27150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van der Houwen T.B., van Laar J., Kappen J.H., van Hagen P.M., de Zoete M.R., van Muijlwijk G.H., Berbers R.M., Fluit A.C., Rogers M., Groot J., et al. Behçet’s disease under microbiotic surveillance? A combined analysis of two cohorts of Behçet’s disease patients. Front. Immunol. 2020;11:1192. doi: 10.3389/fimmu.2020.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.de Paiva C.S., Jones D.B., Stern M.E., Bian F., Moore Q.L., Corbiere S., Streckfus C.F., Hutchinson D.S., Ajami N.J., Petrosino J.F., et al. Altered mucosal microbiome diversity and disease severity in Sjögren syndrome. Sci. Rep. 2016;6:23561. doi: 10.1038/srep23561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li B.Z., Zhou H.Y., Guo B., Chen W.J., Tao J.H., Cao N.W., Chu X.J., Meng X. Dysbiosis of oral microbiota is associated with systemic lupus erythematosus. Arch. Oral Biol. 2020;113:104708. doi: 10.1016/j.archoralbio.2020.104708. [DOI] [PubMed] [Google Scholar]
- 120.Coit P., Mumcu G., Ture-Ozdemir F., Unal A.U., Alpar U., Bostanci N., Ergun T., Direskeneli H., Sawalha A.H. Sequencing of 16S rRNA reveals a distinct salivary microbiome signature in Behçet’s disease. Clin. Immunol. 2016;169:28–35. doi: 10.1016/j.clim.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 121.Chen B., Wang J., Wang Y., Zhang J., Zhao C., Shen N., Yang J., Gai Z., Zhang L. Oral microbiota dysbiosis and its association with Henoch-Schönlein Purpura in children. Int. Immunopharmacol. 2018;65:295–302. doi: 10.1016/j.intimp.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 122.Białowąs K., Radwan-Oczko M., Duś-Ilnicka I., Korman L., Świerkot J. Periodontal disease and influence of periodontal treatment on disease activity in patients with rheumatoid arthritis and spondyloarthritis. Rheumatol. Int. 2020;40:455–463. doi: 10.1007/s00296-019-04460-z. [DOI] [PubMed] [Google Scholar]
- 123.Iordache C., Chirieac R., Ancuta E., Pomirleanu C., Ancuta C. Periodontal disease in patients with ankylosing spondylitis: Myth or reality? Rev. Chim. 2017;68:1660–1664. doi: 10.37358/RC.17.7.5740. [DOI] [Google Scholar]
- 124.Sarkar A., Kuehl M.N., Alman A.C., Burkhardt B.R. Linking the oral microbiome and salivary cytokine abundance to circadian oscillations. Sci. Rep. 2021;11:2658. doi: 10.1038/s41598-021-81420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kessler K., Hornemann S., Rudovich N., Weber D., Grune T., Kramer A., Pfeiffer A., Pivovarova-Ramich O. Saliva Samples as a tool to study the effect of meal timing on metabolic and inflammatory biomarkers. Nutrients. 2020;12:340. doi: 10.3390/nu12020340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen B., Zhao Y., Li S., Yang L., Wang H., Wang T., Shi B., Gai Z., Heng X., Zhang C., et al. Variations in oral microbiome profiles in rheumatoid arthritis and osteoarthritis with potential biomarkers for arthritis screening. Sci. Rep. 2018;8:17126. doi: 10.1038/s41598-018-35473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdelhamid L., Luo X.M. Diet and hygiene in modulating autoimmunity during the pandemic era. Front. Immunol. 2022;12:749774. doi: 10.3389/fimmu.2021.749774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller A.L., Bessho S., Grando K., Tukel C. Microbiome or infections: Amyloid-containing biofilms as a trigger for complex human diseases. Front. Immunol. 2021;12:638867. doi: 10.3389/fimmu.2021.638867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Filoche S., Wong L., Sissons C.H. Oral biofilms: Emerging concepts in microbial ecology. J. Dent. Res. 2010;89:8–18. doi: 10.1177/0022034509351812. [DOI] [PubMed] [Google Scholar]
- 130.Han Y.W., Wang X. Mobile microbiome: Oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013;92:485–491. doi: 10.1177/0022034513487559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tartaglia G.M., Tadakamadla S.K., Connelly S.T., Sforza C., Martín C. Adverse events associated with home use of mouthrinses: A systematic review. Ther. Adv. Drug Saf. 2019;10:2042098619854881. doi: 10.1177/2042098619854881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bescos R., Ashworth A., Cutler C., Brookes Z.L., Belfield L., Rodiles A., Casas-Agustench P., Farnham G., Liddle L., Burleigh M., et al. Effects of Chlorhexidine mouthwash on the oral microbiome. Sci. Rep. 2020;10:5254. doi: 10.1038/s41598-020-61912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim H.S., Woo Chang S., Baek S.H., Han S.H., Lee Y., Zhu Q., Kum K.Y. Antimicrobial effect of alexidine and chlorhexidine against Enterococcus faecalis infection. Int. J. Oral Sci. 2013;5:26–31. doi: 10.1038/ijos.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.James P., Worthington H.V., Parnell C., Harding M., Lamont T., Cheung A., Whelton H., Riley P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017;3:CD008676. doi: 10.1002/14651858.CD008676.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sreenivasan P.K., Haraszthy V.I., Zambon J.J. Antimicrobial efficacy of 0·05% cetylpyridinium chloride mouthrinses. Lett. Appl. Microbiol. 2013;56:14–20. doi: 10.1111/lam.12008. [DOI] [PubMed] [Google Scholar]
- 136.Verspecht T., Herrero E.R., Khodaparast L., Khodaparast L., Boon N., Bernaerts K., Quirynen M., Teughels W. Development of antiseptic adaptation and cross-adapatation in selected oral pathogens in vitro. Sci. Rep. 2019;9:8326. doi: 10.1038/s41598-019-44822-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Decker E.M., Bartha V., Kopunic A., von Ohle C. Antimicrobial efficiency of mouthrinses versus and in combination with different photodynamic therapies on periodontal pathogens in an experimental study. J. Periodontal Res. 2017;52:162–175. doi: 10.1111/jre.12379. [DOI] [PubMed] [Google Scholar]
- 138.Van N., Carr J., Derrick L., Kabani F., Mallonee L. The Oral and Systemic Health Benefits of Arginine. Dimens. Dent. Hyg. 2019;17:40–43. [Google Scholar]
- 139.Kolderman E., Bettampadi D., Samarian D., Dowd S.E., Foxman B., Jakubovics N.S., Rickard A.H. L-arginine destabilizes oral multi-species biofilm communities developed in human saliva. PLoS ONE. 2015;10:e0121835. doi: 10.1371/journal.pone.0121835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tada A., Nakayama-Imaohji H., Yamasaki H., Hasibul K., Yoneda S., Uchida K., Nariya H., Suzuki M., Miyake M., Kuwahara T. Cleansing effect of acidic L-arginine on human oral biofilm. BMC Oral Health. 2016;16:40. doi: 10.1186/s12903-016-0194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koopman J.E., Hoogenkamp M.A., Buijs M.J., Brandt B.W., Keijser B.J., Crielaard W., Ten Cate J.M., Zaura E. Changes in the oral ecosystem induced by the use of 8% arginine toothpaste. Arch. Oral Biol. 2017;73:79–87. doi: 10.1016/j.archoralbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 142.Lin T.H., Lin C.H., Pan T.M. The implication of probiotics in the prevention of dental caries. Appl. Microbiol. Biotechnol. 2018;102:577–586. doi: 10.1007/s00253-017-8664-z. [DOI] [PubMed] [Google Scholar]
- 143.Bustamante M., Oomah B.D., Mosi-Roa Y., Rubilar M., Burgos-Díaz C. Probiotics as an Adjunct Therapy for the Treatment of Halitosis, Dental Caries and Periodontitis. Probiotics Antimicrob. Proteins. 2020;12:325–334. doi: 10.1007/s12602-019-9521-4. [DOI] [PubMed] [Google Scholar]
- 144.Barzegari A., Kheyrolahzadeh K., Khatibi S.M.H., Sharifi S., Memar M.Y., Vahed S.Z. The Battle of Probiotics and Their Derivatives against Biofilms. Infect. Drug Resist. 2020;13:659–672. doi: 10.2147/IDR.S232982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schlagenhauf U., Jockel-Schneider Y. Probiotics in the management of gingivitis and periodontitis. A Review. Front. Dent. Med. 2021;2:708666. doi: 10.3389/fdmed.2021.708666. [DOI] [Google Scholar]
- 146.Goldstep F. Oral Rinses for Periodontal Health: Simplified. [(accessed on 7 February 2022)];Oral Health. 2014 104:40–52. Available online: www.oralhealthgroup.com. [Google Scholar]
- 147.Sälzer S., Rosema N.A., Martin E.C., Slot D.E., Timmer C.J., Dörfer C.E., van der Weijden G.A. The effectiveness of dentifrices without and with sodium lauryl sulfate on plaque, gingivitis and gingival abrasion—A randomized clinical trial. Clin. Oral Investig. 2016;20:443–450. doi: 10.1007/s00784-015-1535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Corbin A., Pitts B., Parker A., Stewart P.S. Antimicrobial penetration and efficacy in an in vitro oral biofilm model. Antimicrob. Agents Chemother. 2011;55:3338–3344. doi: 10.1128/AAC.00206-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Myneni S.R. Effect of baking soda in dentifrices on plaque removal. J. Am. Dent. Assoc. 2017;148:S4–S9. doi: 10.1016/j.adaj.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 150.Hong I., Lee H.G., Keum H.L., Kim M.J., Jung U.W., Kim K., Kim S.Y., Park T., Kim H.J., Kim J.J., et al. Clinical and Microbiological Efficacy of Pyrophosphate Containing Toothpaste: A Double-Blinded Placebo-Controlled Randomized Clinical Trial. Microorganisms. 2020;8:1806. doi: 10.3390/microorganisms8111806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh N. Effectiveness of Green Tea Mouth Rinse over Combination Mouth Rinse in Restoring Salivary pH Post Sugar Exposure in Children. J. Sci. Res. 2020;64:140–143. doi: 10.37398/JSR.2020.640120. [DOI] [Google Scholar]
- 152.Chen Z., Wang Y., Guo C., Li H. The Antibacterial Activity of Natural Product Black Tea Theaflavins on Streptococcus Mutans. Mater. Sci. Eng. 2019;612:022003. doi: 10.1088/1757-899X/612/2/022003. [DOI] [Google Scholar]
- 153.Ben Lagha A., Grenier D. Black tea theaflavins attenuate Porphyromonas gingivalis virulence properties, modulate gingival keratinocyte tight junction integrity and exert anti-inflammatory activity. J. Periodontal Res. 2017;52:458–470. doi: 10.1111/jre.12411. [DOI] [PubMed] [Google Scholar]
- 154.Kong J., Zhang G., Xia K., Diao C., Yang X., Zuo X., Li Y., Liang X. Tooth brushing using toothpaste containing theaflavins reduces the oral pathogenic bacteria in healthy adults. 3 Biotech. 2021;11:150. doi: 10.1007/s13205-021-02699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Piekarz T., Mertas A., Wiatrak K., Rój R., Kownacki P., Śmieszek-Wilczewska J., Kopczyńska E., Wrzoł M., Cisowska M., Szliszka E., et al. The Influence of Toothpaste Containing Australian Melaleuca alternifolia Oil and Ethanolic Extract of Polish Propolis on Oral Hygiene and Microbiome in Patients Requiring Conservative Procedures. Molecules. 2017;22:1957. doi: 10.3390/molecules22111957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Budzyńska A., Wieckowska-Szakiel M., Sadowska B., Kalemba D., Rózalska B. Antibiofilm activity of selected plant essential oils and their major components. Pol. J. Microbiol. 2011;60:35–41. doi: 10.33073/pjm-2011-005. [DOI] [PubMed] [Google Scholar]
- 157.Aires A., Barreto A.S., Semedo-Lemsaddek T. Antimicrobial Effects of Essential Oils on Oral Microbiota Biofilms: The Toothbrush In Vitro Model. Antibiotics. 2020;10:21. doi: 10.3390/antibiotics10010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Guandalini Cunha B., Duque C., Sampaio Caiaffa K., Massunari L., Araguê Catanoze I., Dos Santos D.M., de Oliveira S., Guiotti A.M. Cytotoxicity and antimicrobial effects of citronella oil (Cymbopogon nardus) and commercial mouthwashes on S. aureus and C. albicans biofilms in prosthetic materials. Arch. Oral Biol. 2020;109:104577. doi: 10.1016/j.archoralbio.2019.104577. [DOI] [PubMed] [Google Scholar]
- 159.Benzaid C., Belmadani A., Tichati L., Djeribi R., Rouabhia M. Effect of Citrus aurantium L. Essential Oil on Streptococcus mutans Growth, Biofilm Formation and Virulent Genes Expression. Antibiotics. 2021;10:54. doi: 10.3390/antibiotics10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Horváth B., Balázs V.L., Varga A., Böszörményi A., Kocsis B., Horváth G., Széchenyi A. Preparation, characterisation and microbiological examination of Pickering nano-emulsions containing essential oils, and their effect on Streptococcus mutans biofilm treatment. Sci. Rep. 2019;9:16611. doi: 10.1038/s41598-019-52998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.LeBel G., Haas B., Adam A.A., Veilleux M.P., Lagha A.B., Grenier D. Effect of cinnamon (Cinnamomum verum) bark essential oil on the halitosis-associated bacterium Solobacterium moorei and in vitro cytotoxicity. Arch. Oral Biol. 2017;83:97–104. doi: 10.1016/j.archoralbio.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 162.Dobler D., Runkel F., Schmidts T. Effect of essential oils on oral halitosis treatment: A review. Eur. J. Oral Sci. 2020;128:476–486. doi: 10.1111/eos.12745. [DOI] [PubMed] [Google Scholar]
- 163.Adams S.E., Arnold D., Murphy B., Carroll P., Green A.K., Smith A.M., Marsh P.D., Chen T., Marriott R.E., Brading M.G. A randomised clinical study to determine the effect of a toothpaste containing enzymes and proteins on plaque oral microbiome ecology. Sci. Rep. 2017;7:43344. doi: 10.1038/srep43344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park S.Y., Kim G.J. A biological treasure metagenome: Pave a way for big science. Ind. J. Microbiol. 2008;48:163–172. doi: 10.1007/s12088-008-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sonnenburg J.L., Fischbach M.A. Community health care: Therapeutic opportunities in the human microbiome. Sci. Transl. Med. 2011;3:78ps12. doi: 10.1126/scitranslmed.3001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chiș A., Vulturar R., Andreica S., Prodan A., Miu A.C. Behavioral and cortisol responses to stress in newborn infants: Effects of mode of delivery. Psychoneuroendocrinology. 2017;86:203–208. doi: 10.1016/j.psyneuen.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 167.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017;19:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.