Figure 6.
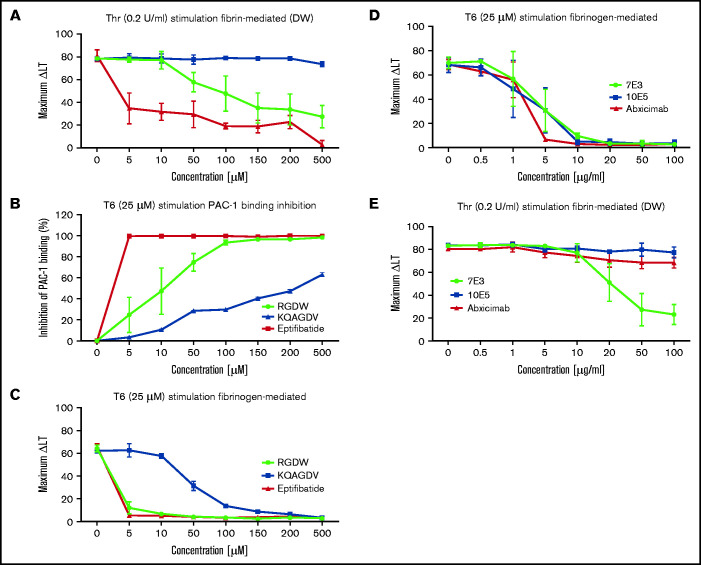
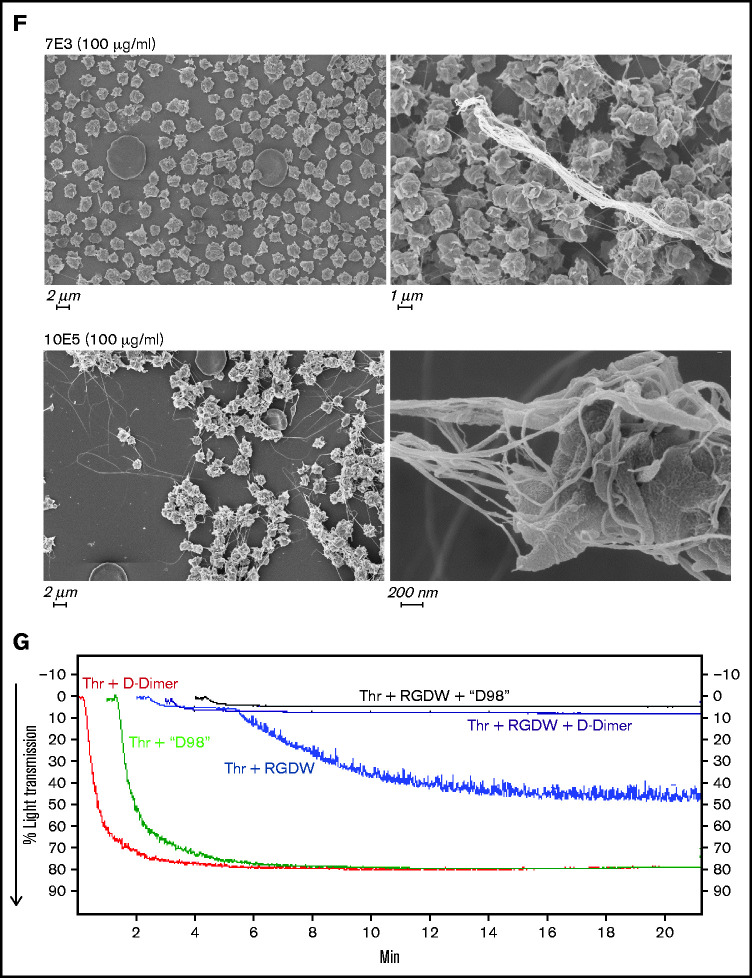
Integrin αIIbβ3 antagonists differentially affect the DW and platelet-polymerizing fibrin interactions, and D-dimer and D98 inhibit the DW. Washed platelets (2 × 108/mL) were treated for 20 minutes at room temperature with increasing doses of the peptides RGDW or KQAGDV, eptifibatide, the mAbs 7E3 or 10E5, or the chimeric mAb 7E3 Fab fragment abciximab. After incubation, platelets were treated with 25 μM T6 (A,D) or 0.2 U/mL Thr (C,E) and stirred in an aggregometer at 37°C. The change in light transmission 15 minutes after adding the agonist was recorded as the maximal ΔLT. (B) In parallel studies, untreated and αIIbβ3 antagonist-treated platelets (2 × 106/mL) were activated with T6 (25 μM) under static conditions without stirring for 5 minutes and incubated with Alexa488-labeled PAC-1 for 20 minutes, after which platelet-associated fluorescence was analyzed by flow cytometry. The data are reported as the mean ± SD inhibition of the geometric mean fluorescence in the absence of each antagonist. (F) SEM of samples of washed platelets 2 minutes after the onset of the DW in the presence of either mAb 7E3 or 10E5. (G) Adding purified D-dimer or D98 at 200 µg/mL to the washed platelets inhibited the DW. One of 3 similar experiments with D-dimer and D98 (mean DW ± SD without and with D-dimer equal 42 ± 6.5 and 5.3 ± 3.2 [P < .005]; mean DW ± SD without and with D98 equal 42 ± 6.5 and 1.7 ± 0.5 [P < .005]).