Abstract
Simple Summary
Hepatocellular carcinoma (HCC) is a major malignancy correlated with many cancer-related deaths. Surgical intervention provides superior long-term survival; however, perioperative mortality is a major concern for clinicians while making treatment decisions, especially for major hepatectomy. Scoring systems for predicting 90-day mortality in patients with HCC undergoing major hepatectomy are not available. By using the stepwise selection of the multivariate Cox proportional hazards model, we divided the patients with HCC receiving major hepatectomy into four risk groups. The Chang Gung-PohAi predictive scoring system showed significant differences in the 90-day mortality rate among the four risk groups (very low risk: 2.42%, low risk: 4.09%, intermittent risk: 17.1%, and high risk 43.6%). The Chang Gung-PohAi predictive scoring system is a promising tool for predicting 90-day perioperative mortality in patients with HCC undergoing major hepatectomy.
Abstract
Purpose: Hepatocellular carcinoma (HCC) is a major malignancy and the common cause of cancer-related deaths. Surgical intervention provides superior long-term survival outcomes; however, perioperative mortality is a major concern for clinicians while making treatment decisions, especially for major hepatectomy. Scoring systems for predicting 90-day mortality in patients with HCC undergoing major hepatectomy are not available. Methods: This study used the Taiwan Cancer Registry Database that is linked to the National Health Insurance Research Database to analyze data of 60,250 patients with HCC who underwent major hepatectomy and determine risk factors to establish a novel predictive scoring system. By using the stepwise selection of the multivariate Cox proportional hazards model, we divided the patients with HCC undergoing major hepatectomy into four risk groups. Results: The Chang Gung-PohAi predictive scoring system exhibited significant differences in the 90-day mortality rate among the four risk groups (very low risk: 2.42%, low risk: 4.09%, intermittent risk: 17.1%, and high risk: 43.6%). Conclusion: The Chang Gung-PohAi predictive scoring system is a promising tool for predicting 90-day perioperative mortality in patients with HCC undergoing major hepatectomy.
Keywords: hepatocellular carcinoma, hepatectomy, 90-day mortality, predictive scoring, overall survival
1. Introduction
Hepatocellular carcinoma (HCC) is the leading primary malignancy of the liver [1,2]. HCC is the seventh most frequently occurring cancer globally and the second most common cause of cancer mortality [1,2]. The incidence rate of HCC is considerably high in Asia and sub-Saharan Africa [3]. Chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections, heavy alcohol consumption, metabolic syndrome, and aflatoxin B are major risk factors for HCC [4,5]. Although the prognosis of HCC is unsatisfactory in all regions of the world, surgical intervention provides favorable outcomes in the very early (Barcelona Clinic Liver Cancer [BCLC] 0) and early (BCLC A) stages of HCC [6]. A study reported that patients beyond the early stage (BCLC stage B) could benefit from liver resection [7]. In the 1980s, liver resection resulted in a relatively high mortality rate, ranging from 10% to 30%, and was thus limited to minor resection [8]. With improvements in patient selection by using the indocyanine green (ICG) test [9], surgical techniques [10], equipment used for parenchymal transection, [11] and postoperative care, the short-term (30-day) mortality rate has substantially improved (<2%) in recent decades [12].
Surgery-related mortality is still a concern for patients and physicians while making decisions regarding the choice of curative treatment, especially major hepatectomy. A future liver remnant (FLR) volume of <26.5% in the normal liver or 31% in the cirrhotic liver after surgery can cause temporary liver failure that may trigger a cascade of complications including massive ascites, hyperbilirubinemia, coagulopathy, hepatorenal syndrome, and even mortality [13]. Technical complications of hepatectomy can result in early postoperative morbidity and mortality [8]. Patients’ outcomes after hospital discharge or within 30 days postoperatively underestimate morbidity and mortality after hepatic resection [14].
The 90-day mortality accounts for the broad spectrum of complications and deaths that can occur late in the postoperative course of patients undergoing liver resection due to systemic cascade effects exerted by deteriorating liver functions including congestive heart failure, renal failure, and sepsis [15]. Several scoring systems are regularly used by physicians to predict the perioperative 90-day mortality risk, including the American Society of Anesthesiologists (ASA) score [16,17,18] and Charlson Comorbidity Index (CCI) [19,20,21,22], especially for patients undergoing orthopedic, head and neck, and urological surgeries [23,24]. With a population of over 23 million, Taiwan has a high prevalence of HCC [25]. However, an appropriate scoring system is not yet available in Taiwan to predict the perioperative 90-day mortality of patients undergoing major hepatectomy. Therefore, we established a predictive model of 90-day mortality by using an easy-to-assess and noninvasive scoring system to rapidly evaluate the risk of perioperative mortality in patients with HCC undergoing hepatectomy.
2. Patients and Methods
2.1. Database
The study cohort was selected from the Taiwan Cancer Registry Database (TCRD, https://twcr.tw/ (accessed on 13 January 2022). We conducted a population-based cohort study by using Taiwan’s National Health Insurance (NHI) Research Database (NHIRD) linked to the TCRD. The NHIRD was established in 1979 and contains the data of 97% of cancer cases in Taiwan [26]. The NHIRD consists of all the medical claims data on disease diagnoses, procedures, drug prescriptions, demographics, and enrollment profiles of all NHI beneficiaries [27]. The NHIRD and TCRD are linked by encrypted patient identifiers. Moreover, because the NHIRD data are linked to the Death Registry, the vital status and cause of death of each patient can be ascertained. The TCRD, which is managed by the Collaboration Center of Health Information Application, contains detailed patient information on various parameters such as clinical stages, surgical procedures, techniques, and chemotherapy regimens [24,28,29,30,31,32].
This study was approved by the Institutional Review Board of Taipei Medical University (TMU-No. 201712019). After the scrambling of identification numbers and the deidentification of personal information, the results are made publicly available for future research purposes.
2.2. Selection of Study Participants
This study evaluated 60,250 patients with HCC who underwent major hepatectomy (clinical stage I–IV) between 1 January 2006, and 31 December 2017. The type of standard major hepatectomy is dependent on the location of lesions and the ability to provide an adequate FLR volume and, for malignant disease, a tumor-negative margin. Standard major hepatectomy involves the resection of two or more liver segments [33]. Patients who underwent wedge resection and one segmental resection were excluded. In addition, patients with Child–Pugh class C disease, Child–Pugh class B disease with an FLR volume of <40%, portal hypertension, and an ICG clearance of >40% at 15 min were excluded. In Taiwan, the majority of HCCs are HBV and HCV related. Usually, the HCC patients have a certain degree of fibrosis and cirrhosis. In this study, patients with portal hypertension were excluded. The diagnosis of portal hypertension based on the presence of ascites or of dilated veins or varices as seen during a physical exam of the abdomen or the anus. Various lab tests (ICG test), pre-operative computer tomography, and endoscopic exams may also be used by the professional surgeons. If the surgeon given a diagnosis of portal hypertension for the HCC patients, patients with severe portal hypertension were excluded in the study because of the high mortality of major liver resection. All of the included patients were aged >20 years. The eligible patients were divided into the following two groups based on their 90-day mortality following major hepatectomy (the index date was designated as the date on which the patients underwent major hepatectomy): 90-day mortality and 90-day survival.
2.3. Statistical Analysis
All statistical analyses were performed using SAS for Windows (version 9.4; SAS Institute, Cary, NC). A p value of ≤0.05 was considered statistically significant. Essential demographic characteristics, namely sex and age, were categorized. Patient age was determined according to the index date. Accordingly, the patients were divided into six age groups (20–29, 30–39, 40–49, 50–59, 60–69, and ≥70 years). The variables of interest were demographic characteristics; BCLC classification; American Joint Committee on Cancer (AJCC) tumor, node, and metastasis stages; presence of any other cancer, other metastatic cancers, leukemia, or lymphoma; and comorbidities. According to previous studies, comorbidities were determined from the NHIRD or TCRD [24,34,35,36]. Patients with diabetes mellitus (DM), pneumonia, chronic obstructive pulmonary disease (COPD), HBV infection, HCV infection, angina, heart valve dysfunction, sepsis, heart failure, disseminated intravascular coagulation (DIC), adult respiratory distress syndrome (ARDS), aortic aneurysm, peripheral vascular disease, peptic ulcer disease, dementia, chronic pulmonary disease, connective tissue disease, mild liver disease, moderate or severe liver disease, hemiplegia, coronary artery disease, myocardial infarction (MI), an implanted pacemaker, hypertension, chronic kidney disease (CKD), moderate or severe renal disease, end-stage renal disease, cerebral vascular accident, and transient ischemic attack were examined. Other cancer statuses and comorbid conditions reported >1 year before the index date were not included to ensure relevance. On the basis of the International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes, comorbidities were identified if patients received a positive diagnosis in a single admission or had two or more repeated visits to outpatient departments within 1 year. The chi-square test was used to compare demographic characteristics, HCC staging, other cancer statuses, and comorbidities between the mortality and survival groups (Table 1).
Table 1.
Demographic Characteristics of the 90-Day Mortality and 90-Day Survival Groups of Patients with Hepatocellular Carcinoma Undergoing Major Hepatectomy.
| Characteristics | 90-Day Survival No. (%) |
90-Day Mortality No. (%) |
p Value | |
|---|---|---|---|---|
| n | 57,525 (95.5%) | 2725 (4.5%) | ||
| Sex | Female | 20,859 (36.3) | 885 (32.5) | <0.001 |
| Male | 36,666 (63.7) | 1840 (67.5) | ||
| Age, mean (SD) | 58.39 (13.72) | 61.61 (16.43) | <0.001 | |
| Age (years) | 20–29 | 1653 (2.9) | 172 (6.3) | <0.001 |
| 30–39 | 3499 (6.1) | 112 (4.1) | ||
| 40–49 | 8402 (14.6) | 263 (9.7) | ||
| 50–59 | 15,023 (26.1) | 441 (16.2) | ||
| 60–69 | 16,316 (28.4) | 719 (26.4) | ||
| ≥70 | 12,632 (22.0) | 1018 (37.4) | ||
| Comorbidities | ||||
| Diabetes mellitus | Yes | 12,731 (22.1) | 812 (29.8) | <0.001 |
| Pneumonia | Yes | 2859 (5.0) | 320 (11.7) | <0.001 |
| COPD | Yes | 2686 (4.7) | 224 (8.2) | <0.001 |
| Hepatitis B | Yes | 18,659 (32.4) | 492 (18.1) | <0.001 |
| Hepatitis C | Yes | 10,065 (17.5) | 447 (16.4) | 0.149 |
| Heart valve dysfunction | Yes | 976 (1.7) | 56 (2.1) | 0.182 |
| Sepsis | Yes | 2278 (4.0) | 505 (18.5) | <0.001 |
| Heart failure | Yes | 1330 (2.3) | 151 (5.5) | <0.001 |
| Disseminated intravascular coagulation | Yes | 27 (0.0) | 46 (1.7) | <0.001 |
| ARDS | Yes | 41 (0.1) | 25 (0.9) | <0.001 |
| Aortic aneurysm | Yes | 96 (0.2) | 15 (0.6) | <0.001 |
| Peripheral vascular disease | Yes | 566 (1.0) | 43 (1.6) | 0.003 |
| Peptic ulcer disease | Yes | 12,853 (22.3) | 775 (28.4) | <0.001 |
| Dementia | Yes | 511 (0.9) | 59 (2.2) | <0.001 |
| Chronic pulmonary disease | Yes | 2617 (4.5) | 179 (6.6) | <0.001 |
| Connective tissue disease | Yes | 512 (0.9) | 20 (0.7) | 0.455 |
| Mild liver disease | Yes | 27,019 (47.0) | 1100 (50.4) | <0.001 |
| Hemiplegia | Yes | 1456 (2.5) | 123 (4.5) | <0.001 |
| Moderate or severe liver disease | Yes | 8362 (14.5) | 413 (17.2) | <0.001 |
| Coronal arterial disease | Yes | 4390 (7.6) | 278 (10.2) | <0.001 |
| Myocardial infarction | Yes | 164 (0.3) | 42 (1.5) | <0.001 |
| HTN | Yes | 20,474 (35.6) | 993 (36.4) | 0.377 |
| Angina | Yes | 868 (1.5) | 72 (2.6) | <0.001 |
| CKD | Yes | 250 (0.4) | 26 (1.0) | <0.001 |
| Moderate or severe renal disease | Yes | 1622 (2.8) | 425 (15.6) | <0.001 |
| End-stage renal disease | Yes | 1904 (3.3) | 175 (6.4) | <0.001 |
| Cerebral vascular accident | Yes | 1738 (3.0) | 165 (6.1) | <0.001 |
| Transient ischemic attack | Yes | 846 (1.5) | 89 (3.3) | <0.001 |
| Implanted pacemaker | Yes | 11 (0.0) | 3 (0.1) | 0.016 |
| Cancer status | ||||
| Any other cancers | Yes | 14,632 (25.4) | 746 (27.4) | 0.025 |
| Leukemia | Yes | 44 (0.1) | 3 (0.1) | 0.793 |
| Lymphoma | Yes | 194 (0.3) | 17 (0.6) | 0.021 |
| Metastatic solid tumor for other cancers | Yes | 1229 (2.1) | 72 (2.6) | <0.001 |
| Surgical techniques | 0.948 | |||
| Open | 54,591 (94.9) | 2589 (95.0) | ||
| Laparoscopic surgery | 2934 (5.1) | 136 (5.0) | ||
| Hepatocellular cell carcinoma stages | ||||
| BCLC classification | 36,768 (63.9) | 1970 (72.3) | <0.001 | |
| 0 | 11,038 (19.2) | 217 (8.0) | ||
| A | 3312 (5.8) | 134 (4.9) | ||
| B | 3544 (6.2) | 249 (9.1) | ||
| C | 2863 (5.0) | 155 (5.7) | ||
| AJCC Clinical T stages | 36,767 (63.9) | 1970 (72.3) | <0.001 | |
| cT1 | 11,143 (19.4) | 229 (8.4) | ||
| cT2 | 3569 (6.2) | 151 (5.5) | ||
| cT3 | 4496 (7.8) | 273 (10.0) | ||
| cT4 | 1550 (2.7) | 102 (3.7) | ||
| Clinical N stages | 36,767 (63.9) | 1970 (72.3) | <0.001 | |
| cN0 | 17,957 (31.2) | 620 (22.8) | ||
| cN1 | 2801 (4.9) | 135 (5.0) | ||
| Clinical M stages | 36,767 (63.9) | 1970 (72.3) | <0.001 | |
| cM0 | 18,265 (31.8) | 636 (23.3) | ||
| cM1 | 2493 (4.3) | 119 (4.4) |
BCLC, Barcelona Clinic Liver Cancer; COPD, chronic obstructive pulmonary disease; ARDS, adult respiratory distress syndrome; HTN, hypertension; CKD, chronic kidney disease; SD, standard deviation; T stage, Tumor stage; N stage, nodal stage; M stage, metastatic stage; AJCC, American Joint Committee on Cancer.
All significant factors were identified to construct the Chang Gung-PohAi Major Hepatectomy Mortality Predictive Scoring System for 90-day mortality in patients with HCC undergoing major hepatectomy. Univariate and multivariate Cox proportional hazards models were used to determine the hazard ratio (HR) and 95% confidence interval (CI) of each factor (Table 2 and Table 3, respectively). The stepwise selection method was used to select all factors that significantly predicted 90-day mortality (Table 3). A forward stepwise selection method was employed to select all variables that exerted significant effects (p < 0.05) on the survival duration of the patients. Variables with a coefficient of >0 or an adjusted HR (aHR) of >1 were selected as risk factors to construct the Chang Gung-PohAi-Major Hepatectomy Mortality Predictive Scoring System by addition of points according to the aHR. The stepwise method modifies the forward selection technique such that effects already existing in the model do not necessarily remain there. During the stepwise selection method, duplicate entry and removal approaches were used for the forward selection and backward elimination to evaluate the contribution of effects as they were added to or removed from a model. Notably, the “minimum F-to-enter” was used to add or remove a variable. The most favorable model was chosen on the basis of the information criterion. Factors with an aHR of ≥1 were considered risk factors. The risk point for each risk factor was defined as the highest integer less than or equal to its corresponding aHRs in stepwise regression [23]. The patients were divided into four risk groups according to their risk scores (Table 4). The patients with high risk scores were predicted to have increased 90-day mortality following major hepatectomy. The long-term mortality rate determined using the Chang Gung-PohAi-Major Hepatectomy Mortality Predictive Scoring System was evaluated using the Kaplan–Meier method, and differences among the risk groups were determined using the log-rank test. A two-tailed p value of <0.05 was considered statistically significant.
Table 2.
All-Cause 90-Day Mortality Risk Assessment Using a Multivariate Cox Proportional Hazards Model in Patients with Hepatocellular Carcinoma Undergoing Major Hepatectomy.
| Factor | aHR * | 95% CI | p Value |
|---|---|---|---|
| Age (years) | |||
| 20–29 | Reference | ||
| 30–39 | 0.745 | 0.302, 1.837 | 0.5228 |
| 40–49 | 0.756 | 0.328, 1.746 | 0.5129 |
| 50–59 | 0.857 | 0.379, 1.938 | 0.7103 |
| 60–69 | 1.139 | 0.506, 2.563 | 0.7536 |
| ≧70 | 2.117 | 0.944, 4.749 | 0.0689 |
| Sex | |||
| Female | Reference | ||
| Male | 1.015 | 0.865, 1.191 | 0.8537 |
| Comorbidities | |||
| Diabetes mellitus | 1.689 | 1.458, 1.957 | <0.001 |
| Pneumonia | 2.896 | 2.338, 3.588 | <0.001 |
| COPD | 1.903 | 1.481, 2.445 | <0.001 |
| Hepatitis B | 1.021 | 0.429, 1.585 | 0.4010 |
| Hepatitis C | 0.975 | 0.824, 1.153 | 0.7632 |
| Heart valve dysfunction | 1.817 | 1.219, 2.706 | 0.0033 |
| Sepsis | 8.688 | 7.275, 10.376 | <0.001 |
| Heart failure | 2.898 | 2.202, 3.813 | <0.001 |
| Disseminated intravascular coagulation | 35.258 | 15.789, 78.733 | <0.001 |
| ARDS | 3.948 | 0.556, 28.059 | 0.1699 |
| Aortic aneurysm | 2.278 | 0.734, 7.064 | 0.1540 |
| Peripheral vascular disease | 2.376 | 1.54, 3.667 | <0.001 |
| Peptic ulcer disease | 1.53 | 1.31, 1.786 | <0.001 |
| Dementia | 2.649 | 1.699, 4.13 | <0.001 |
| Chronic pulmonary disease | 1.353 | 1.004, 1.822 | 0.0471 |
| Connective tissue disease | 1.086 | 0.361, 1.799 | 0.5979 |
| Mild liver disease | 1.050 | 0.650, 1.085 | 0.7461 |
| Hemiplegia | 2.291 | 1.700, 3.087 | <0.001 |
| Moderate or severe liver disease | 1.169 | 0.981, 1.394 | 0.0804 |
| Coronal arterial disease | 1.197 | 0.944, 1.517 | 0.1372 |
| Myocardial infarction | 6.275 | 3.824, 10.296 | <0.001 |
| HTN | 1.144 | 0.991, 1.321 | 0.0661 |
| Angina | 2.058 | 1.445, 2.933 | <0.001 |
| CKD | 2.439 | 1.307, 4.551 | 0.0051 |
| Moderate or severe renal disease | 7.185 | 5.98, 8.633 | <0.001 |
| End-stage renal disease | 2.051 | 1.579, 2.664 | <0.001 |
| Cerebral vascular accident | 2.251 | 1.718, 2.949 | <0.001 |
| Transient ischemic attack | 2.516 | 1.792, 3.532 | <0.001 |
| Implanted pacemaker | 1.000 | 0.999, 1.001 | 0.9990 |
| Cancer status | |||
| Any other cancers | 1.029 | 0.858, 1.235 | 0.7565 |
| Leukemia | 1.000 | 0.991, 1.001 | 0.9977 |
| Lymphoma | 2.672 | 0.376, 18.993 | 0.3259 |
| Other metastatic solid tumor | 1.525 | 1.295, 1.797 | <0.001 |
| Surgical techniques | |||
| Open | Reference | ||
| Laparoscopic surgery | 0.996 | 0.782, 3.124 | 0.8697 |
| Hepatocellular cell carcinoma stages | |||
| BCLC classification 0 | Reference | ||
| BCLC classification A | 2.043 | 1.647, 2.534 | <0.001 |
| BCLC classification B | 3.490 | 2.908, 4.187 | <0.001 |
| BCLC classification C | 2.705 | 2.201, 3.325 | <0.001 |
| AJCC cT1 | Reference | ||
| AJCC cT2 | 2.042 | 1.663, 2.508 | <0.001 |
| AJCC cT3 | 2.898 | 2.431, 3.455 | <0.001 |
| AJCC cT4 | 3.126 | 2.475, 3.948 | <0.001 |
| AJCC cN0 | Reference | ||
| AJCC cN1 | 3.015 | 1.471, 4.579 | <0.001 |
| AJCC cM0 | Reference | ||
| AJCC cM1 | 1.361 | 1.119, 1.655 | 0.002 |
BCLC, Barcelona Clinic Liver Cancer; COPD, chronic obstructive pulmonary disease; ARDS, adult respiratory distress syndrome; HTN, hypertension; CKD, chronic kidney disease; SD, standard deviation; T stage, tumor stage; N stage, nodal stage; M stage, metastatic stage; AJCC, American Joint Committee on Cancer; HR, hazard ratio; CI, confidence interval. * All variables were used in multivariate analysis.
Table 3.
Stepwise Selection of the Multivariate Cox Proportional Hazards Model for All-Cause 90-Day Mortality in Patients with Hepatocellular Carcinoma Undergoing Major Hepatectomy.
| Factor | HR * | Score |
|---|---|---|
| Age: 30–39 years | 0.854 | 0 |
| Age: 40–49 years | 0.974 | 0 |
| Age: 50–59 years | 0.817 | 0 |
| Age: 60–69 years | 0.967 | 0 |
| Age: ≧70 years | 1.462 | 1 |
| Comorbidities | ||
| Diabetes mellitus | 1.426 | 1 |
| Pneumonia | 1.703 | 2 |
| Hepatitis B | 0.910 | 0 |
| Sepsis | 4.762 | 5 |
| Heart failure | 1.604 | 2 |
| Disseminated intravascular coagulation | 14.055 | 14 |
| Peptic ulcer disease | 1.336 | 1 |
| Dementia | 1.703 | 2 |
| Myocardial infarction | 3.624 | 4 |
| HTN | 0.998 | 0 |
| Moderate or severe renal disease | 4.477 | 4 |
| Cerebral vascular accident | 1.48 | 1 |
| Transient ischemic attack | 1.582 | 2 |
| Cancer status | ||
| Any other cancers | 0.942 | 0 |
| Other metastatic solid tumor | 1.421 | 1 |
| Hepatocellular cell carcinoma status | ||
| BCLC classification A | 1.929 | 2 |
| BCLC classification B | 3.557 | 4 |
| BCLC classification C | 4.024 | 4 |
| AJCC cN1 | 3.272 | 3 |
BCLC, Barcelona Clinic Liver Cancer; COPD, chronic obstructive pulmonary disease; ARDS, adult respiratory distress syndrome; HTN, hypertension; CKD, chronic kidney disease; SD, standard deviation; T stage, tumor stage; N stage, nodal stage; M stage, metastatic stage; AJCC, American Joint Committee on Cancer; HR, hazard ratio. * All variables were used in multivariate analysis.
Table 4.
All-Cause 90-Day Mortality Assessment Using Taiwan-Major Hepatectomy Mortality Predictive Scoring System for Hepatocellular Carcinoma.
| Chang Gung-Poh Ai Cumulative Score | Survivors | Deaths | 90-Day Mortality Rate after Major Hepatectomy |
|---|---|---|---|
| 0 | 17,301 | 422 | 2.44% |
| 1 | 16,552 | 445 | 2.69% |
| 2 | 8651 | 337 | 3.90% |
| 3 | 4067 | 207 | 5.09% |
| 4 | 3398 | 186 | 5.47% |
| 5 | 4287 | 250 | 5.83% |
| 6 | 2719 | 224 | 8.24% |
| 7 | 1257 | 147 | 11.69% |
| 8 | 676 | 100 | 14.79% |
| 9 | 438 | 94 | 21.46% |
| 10 | 344 | 103 | 29.94% |
| 11 | 218 | 60 | 27.52% |
| 12 | 112 | 34 | 30.36% |
| 13 | 71 | 23 | 32.39% |
| 14 | 61 | 32 | 52.46% |
| 15 | 25 | 13 | 52.00% |
| 16 | 24 | 17 | 70.83% |
| 17 | 6 | 3 | 50.00% |
| 18+ | 43 | 28 | 65.12% |
3. Results
3.1. Demographic Characteristics of Patients with HCC Receiving Major Hepatectomy
We compared the basic data and comorbidities between the 90-day survival and 90-day mortality groups. Of the 60,250 patients enrolled in this study, 2725 (1840 [67%] men; mean [standard deviation, SD] age, 61.61 [16.43] years) died before reaching the 90-day threshold, whereas 57,525 patients (36,666 [63.7%] men; mean [SD] age, 58.39 [13.72] years) survived for more than 90 days. The 90-day mortality rate of the patients with HCC after major hepatectomy was 4.5%. The male to female ratio (63.7:36.3 vs. 67.5:32.5, p < 0.01), mean [SD] age (58.39 [13.72] vs. 61.61 [16.43] years, p < 0.01), and some comorbidities (e.g., DM, pneumonia, sepsis, heart failure, cerebral vascular disease, chronic renal disease, different stages of liver disease, HBV infection, different BCLC stages, AJCC clinical stages, and other cancer statuses) significantly differed between the 90-day survival and 90-day mortality groups (Table 1). By contrast, the proportion of the patients with HCV infection and hypertension did not significantly differ between the groups.
3.2. 90-Day Mortality Risk Assessment after Major Hepatectomy
We used the multivariate Cox proportional hazards model to analyze all the causes of 90-day mortality. Risk factors for 90-day mortality determined using the multivariate Cox proportional hazards model are listed in Table 2. After dividing the patients into six age groups and using the youngest age group (20–39 years) as reference, we observed no significant effect of any age group on the 90-day mortality outcome. Furthermore, no significant effect of sex on the 90-day mortality outcome was noted (aHR, 1.015; 95% CI, 0.865–1.191, p = 0.8537). Comorbidities, namely DM, pneumonia, COPD, heart valve dysfunction, sepsis, heart failure, DIC, peripheral vascular disease, peptic ulcer disease, dementia, chronic pulmonary disease, hemiplegia, myocardial infarction, angina, CKD, cerebral vascular, and BCLC and AJCC stages, were determined to be significant independent risk factors for 90-day mortality after major hepatectomy.
3.3. Stepwise Selection for 90-Day Mortality after Major Hepatectomy
Table 3 lists all significant factors determined by applying the stepwise method in the multivariate model for variable selection. On the basis of the HR, each different risk factor was assigned a score. After the stepwise selection of the multivariate Cox proportional hazards model for 90-day mortality in the patients with HCC receiving major hepatectomy, age ≥70 years (aHR: 1.462, score: 1); DM (aHR: 1.426, score: 1); pneumonia (aHR: 1.703, score: 2); sepsis (aHR: 4.762, score: 5); heart failure (aHR: 1.604, score: 2); DIC (aHR: 14.055, score: 14); peptic ulcer (aHR: 1.336, score: 1); dementia (aHR: 1.703, score: 2); myocardial infarction (aHR: 3.624, score: 4); moderate or severe renal disease (aHR: 4.477, score: 4); cerebral vascular accident (aHR: 1.48, score: 1); transient ischemic attack (aHR: 1.582, score: 2); other metastatic solid tumor (aHR: 1.421, score: 1); BCLC stage A, B, and C (aHR: 1.020, 3.557, and 4.024, respectively; score = 2, 4, and 4, respectively); and AJCC cN1 (aHR: 3.310, score: 3) were determined as significant independent risk factors for 90-day mortality after hepatectomy.
3.4. 90-Day Mortality Assessment Using the Chang Gung-PohAi Mortality Predictive Scoring System
The risk score (Chang Gung-PohAi cumulative score) was calculated on the basis of the accumulation of risk factors. The proportion of the patients who died within 90 days consistently increased with the accumulation of risk scores (e.g., score, 90-day mortality [0, 2.44%], [7, 11.69%], [12, 30.36%], and [18+, 65.12%]). The results of 90-day mortality assessment obtained using the Chang Gung-PohAi mortality predictive scoring system are presented in Table 4. We categorized the patients into very low risk (score = 0), low risk (score = 1–6), moderate risk (score = 7–11), and high risk (score ≥ 12) groups.
3.5. Kaplan–Meier Survival Curve for 90-Day Mortality Determined Using Chang Gung-PohAi, American Society of Anesthesiologists, or CCI Scores
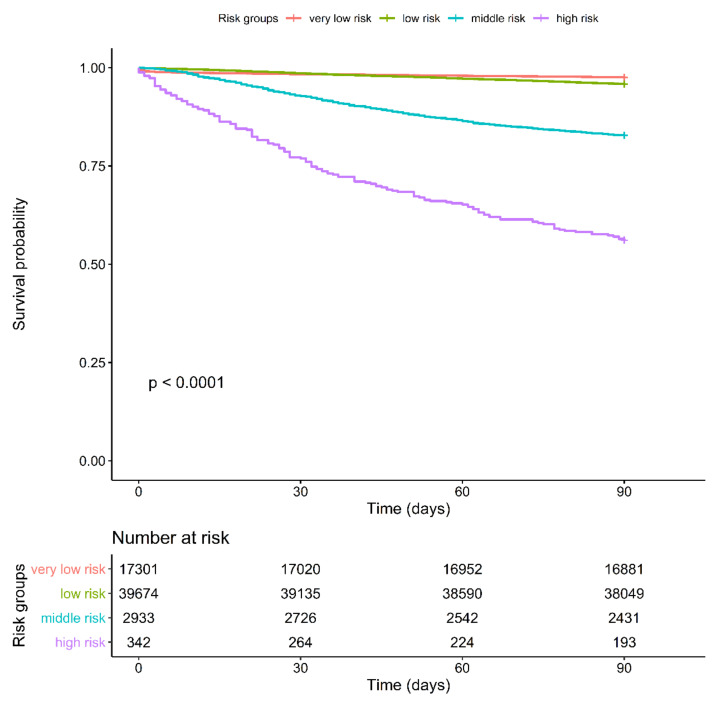
As shown in Figure 1, the 90-day mortality did not significantly differ between the very-low-risk and low-risk groups. By contrast, the 90-day mortality significantly differed between the moderate-risk and high-risk groups. The American Society of Anesthesiologists (ASA) score [16] (ASA levels 1 and 2 are defined as low risk and ASA levels 3 and 4 are defined as high risk) and CCI score [19] (score: 0–5, low risk and score: 6+, high risk) are applied in some cancer surgeries to predict perioperative mortality (Tables S1 and S2). Therefore, we examined our data by using the ASA score (Figure S1) and CCI score (Figure S2). Although both the systems showed significant differences (ASA score, P = 0.00059; CCI score, p < 0.0001), the graph failed to show a distinct separation between the low-risk and high-risk groups in both the scoring systems. According to the risk score determined using the Chang Gung-PohAi predictive scoring system, the patients were divided into four groups: very low risk (score: 0), low risk (score: 1–6), intermittent risk (score: 7–11), and high risk (score: 12–18+). As shown in Figure 1, the 90-day mortality rate significantly differed among the four risk groups (very low risk: 2.42%, low risk: 4.09%, intermittent risk: 17.1%, and high risk: 43.6%). Hence, compared with the CCI and ASA scoring systems, the predictive model of 90-day mortality after hepatectomy was more favorable (Figures S1 and S2).
Figure 1.
Kaplan–Meier Survival Curve for 90-Day Mortality for Four Chang Gung-PohAi-Major Hepatectomy Mortality Predictive Scoring System Groups. Note: p value (log-rank test) <0.0001.
3.6. Kaplan–Meier Curve for 5-Year Overall Survival Determined Using Chang Gung-PohAi, ASA, and CCI Scores
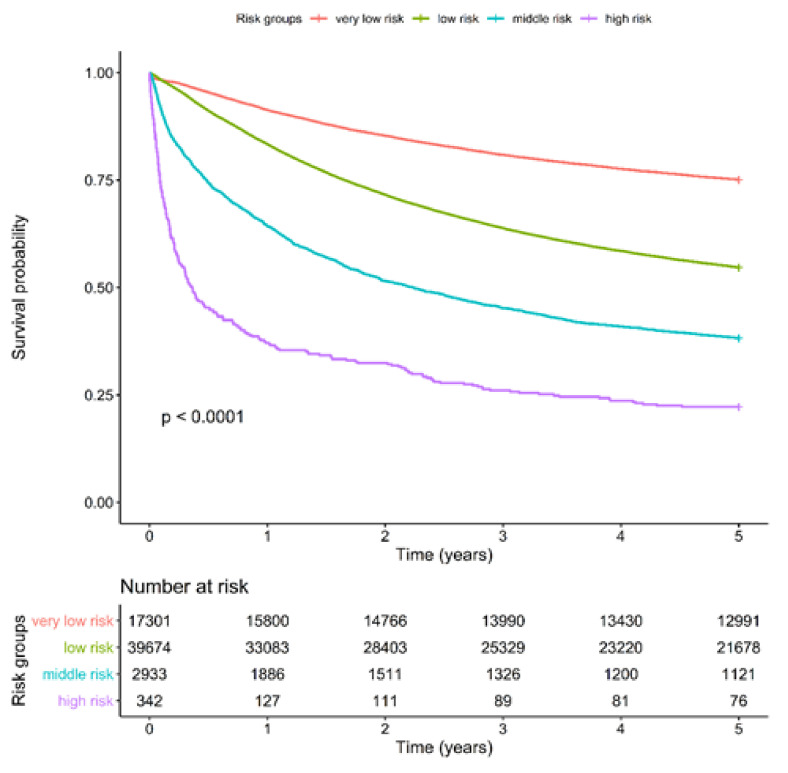
We examined whether our scoring system can predict long-term survival. A significant 5-year survival difference was observed between the very-low-risk (75.1%) and low-risk (54.6%) groups. The other two risk groups both exhibited an early 90-day mortality trend (intermittent risk: 38.2% and high risk: 22.2%; Figure 2). The ASA scoring system exhibited a slight difference in 5-year overall survival between levels 1 and 2 and levels 3 and 4 (Figure S3). The CCI scoring system exhibited significant 5-year overall survival differences between the Gr 0–5 and Group 6+ (Figure S4).
Figure 2.
Kaplan–Meier 5-Year Survival Curve for Long-Term Mortality Rate of Four Chang Gung-PohAi-Major Hepatectomy Mortality Predictive Scoring System Groups. Note: p (log-rank test <0.0001).
The Chang Gung-PohAi predictive scoring system predicted not only the perioperative 90-day mortality risk but also the long-term survival outcomes of the four risk groups of the patients undergoing major hepatectomy. Furthermore, the prediction of risks and outcomes by this novel scoring system was more satisfactory than that of the ASA and CCI scoring systems for the patients with HCC undergoing receiving major hepatectomy.
4. Discussion
To date, an easy-to-use and a noninvasive scoring system for predicting the perioperative safety of patients undergoing major hepatectomy is not available. HCC is the leading cause of cancer deaths in China and Taiwan [37,38]. Resection of HCC with a free margin is the most vital curative treatment [39] rather than liver transplantation, especially in areas with a high prevalence of HCC [40]. However, a high 90-day mortality rate (approximately 4%) is still observed in patients undergoing major hepatectomy in Taiwan (Table 1) after preoperative evaluation performed using the Child–Pugh classification system, FLR volume examination, and ICG test at 15 min. Hence, an easy-to-use predictive tool for 90-day mortality can be valuable for patients undergoing hepatectomy in areas with a high prevalence of HCC because liver transplantation with a sufficient donor liver is impossible for most of the patients with HCC in such areas. Hepatectomy is still the major treatment modality for resecting HCC in areas with a high prevalence of HCC. Traditionally, the preoperative ICG test and liver function evaluation using Child–Pugh and liver fibrotic scores have been widely used for preoperative evaluation [41,42]. Despite the preoperative liver function evaluation, the 90-day mortality was still high in Taiwan (Table 1). Therefore, we conducted this study to establish a predictive scoring system to easily evaluate 90-day mortality that can be helpful in shared decision-making by patients with HCC and surgeons for choosing future treatment modalities. The scoring system established in this study predicted not only the 90-day mortality but also the long-term survival of the patients with HCC.
The Child–Pugh classification system has been the most widely used for assessing liver function and is crucial for predicting outcomes after hepatectomy [43]. The Fibrosis-4 (FIB-4) index is closely associated with liver fibrosis and cirrhosis [44]. The FIB-4 index effectively predicted the outcomes of patients with HCC after hepatectomy [44]. However, the FIB-4 index score predicted only the liver cirrhosis condition as the long-term outcome following hepatectomy [44]. In our study, although the prediction of the CCI scoring system was more favorable than that of the ASA scoring system, the liver fibrosis condition was not included in either of these two systems. The Chang Gung-PohAi predictive scoring system included not only the liver cirrhosis grade but also other crucial comorbidities and age. We demonstrated that by using this novel scoring system, the patients could be divided into four risk groups of 90-day mortality after major hepatectomy. Moreover, this scoring system could be used to predict the long-term survival of the patients with HCC undergoing hepatectomy.
Previously, scoring systems, such as the Child–Pugh and FIB-4 scores, based on liver function and the remaining liver volume after liver resection were used to examine mortality. In patients with less severe disease, the degree to which the underlying liver disease constitutes an absolute versus relative contraindication to hepatic resection depends upon the anticipated volume of liver remaining after resection (FLR volume) [13,45], the presence of medical comorbidities, and resources available in the event of perioperative liver failure such as the availability and proximity of liver transplantation [46]. In addition, the model for end-stage liver disease scores does not directly affect decision-making related to liver resection but may be useful in counseling a patient when choosing between liver resection and transplant [47]. For patients with normal liver function, an FLR volume of <20% increases the risk of liver failure and death following major hepatic resection [48]. Patients with mild-to-moderate underlying functional liver disease have increased risks of liver failure and death if the future liver remnant is inadequate [49]. These aforementioned evaluation tools using liver function and FLR volume have been used for a long time in Taiwan. Although all patients with HCC receiving hepatectomy in our study were evaluated using these aforementioned tools, a 90-day mortality rate of 4% was still noted in our study. The most crucial reasons might be the underlying comorbidities and age that were not considered in the evaluation.
Although FLR volume is a major factor that affects perioperative mortality in major hepatectomy, more complex systemic factors, such as chronic heart disease, cerebral vascular disease, and chronic renal function, can compete the risk of perioperative mortality [50,51]. However, no firm guidelines define what constitutes “inadequate” for specific populations receiving hepatectomy. The ASA score guide was developed by Dr. Meyer in 1941 [52] and amended in 1980. The ASA classification for a particular patient is based on systemic diseases. The extent of this disease is evident from patients’ medical history and medication list and the degree of limitation that the disease causes in patients’ everyday life. The rate of postoperative complications was found to be closely related to the ASA class (ASA score I = 0.41/1000; scores IV and V = 9.6/1000) and with emergency surgeries (ASA I = 1/1000 increases to 26.5/1000 in classes IV and V) [53]. The ASA scoring system has been widely used by clinicians in several surgical fields for determining the perioperative mortality risk. However, limited evidence indicates that the ASA score can be used to predict outcomes in patients undergoing liver resection [53]. Meanwhile, the CCI score accounts for most of the major medical comorbidities and demonstrated a reliable short-term predictive ability for postoperative patients in several surgical fields (e.g., hip surgery and urological surgery) [54]. The modified CCI score that recalibrated the weighting system more accurately predicted survival after liver transplantation [55]. However, no study has reported that the CCI score has a reliable predictive value for perioperative mortality after major hepatectomy.
The NHIRD encompasses the health information of 99% of the Taiwanese population since 1995 [56]. Research based on the NHI database provides valuable data regarding cancer diagnosis, epidemiology, outcome, and treatment [56]. The TCRD in Taiwan is a compulsory system that requires all hospitals treating patients with cancer to provide valid clinical, laboratory, imaging, pathology, and personal data to the Ministry of Health and Welfare [57]. The data were validated and generally consistent in both the databases [58]. By using accurate, large-scale, and complete data, we established the reliable Chang Gung-PohAi predictive scoring system that exhibited a significant difference in 90-day mortality among low-risk, mid-risk, and high-risk groups. By contrast, the ASA and CCI scoring systems failed to show a significant difference in survival among patients with different risks in 90 days.
There are some limitations in our study. First, based on the current findings, the clinical application of this study is limited currently because of lack of validation set. Moreover, the scoring system refers only for patients with Child Pugh A and with Child Pugh B with an FLR < 40%. However, our study included a large sample size of patients to develop a rapid assessment scoring system. Second, some comorbidities, as DIC or sepsis, have a high impact on the proposed score. However, DIC or sepsis should be effectively treated before considering a patient for major hepatectomy. It is supposed that DIC or sepsis have been be effectively treated before considering a patient for major hepatectomy. However, the patients with DIC or sepsis as the comorbidities mean the health condition or their heath environment were poorer than the patients without DIC or sepsis. Therefore, the patients with DIC or sepsis contributed to poor health conditions associated with higher mortality after major liver resection. Therefore, the predictive scoring system could give the rapid scoring to predict the 90-Day mortality after major liver resection using the easy access before surgery. Third, patients in both groups had a metastatic solid tumor and underwent hepatectomy. Patients with other metastatic solid tumor for other cancers have been under controlled in the study. For example, patients with lung adenocarcinoma with solitary brain metastasis status post ablation by stereotactic radiosurgery received hepatectomy, sequentially. It is possible for patients with a metastatic solid tumor and who underwent hepatectomy, because the ablation techniques of limited metastasis have been improving, whatever radiofrequency ablation, and microwave thermal ablation, high-intensity focused ultrasound, stereotactic radiosurgery or cryotherapy. Around 2% patients with metastatic solid tumor for other cancers receiving hepatectomy were possible, if the limited metastasis could also be removed by surgery, or other ablation therapy. Fourth, 5% of patients underwent major hepatectomy in BCLC C. If patients with BCLC stage C could pass the exclusion criteria, physicians would choose hepatectomy for them with curative-intent treatment. Therefore, 5% of patients with BCLC stage C might be possible. Because hepatectomy for patients with BCLC stage C might be a real-world clinical practice, we thought exclusion of BCLC stage C patients would be unreasonable. Consider the BCLC stage C as a part of a scoring system could be helpful to predict the 90-Day mortality after hepatectomy.
5. Conclusions
The novel Chang Gung-PohAi predictive scoring system, which considers comprehensive patients’ tumor-related factors, age, and systemic comorbidities, is a promising tool for predicting 90-day perioperative mortality and long-term survival in patients with HCC undergoing major hepatectomy.
Acknowledgments
Lo-Hsu Medical Foundation, Lotung Poh-Ai Hospital, supports Szu-Yuan Wu’s work (Funding Number: 11001, 11010, 11013 and 11103).
Abbreviations
HCC: Hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; BCLC, Barcelona Clinic Liver Cancer; ICG, indocyanine green; FRL, future remnant liver; ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; TCRD, Taiwan Cancer Registry Database; NHI, National Health Insurance; NHIRD, National Health Insurance Research Data; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease; DIC, disseminated intravascular coagulation; ARDS, adult respiratory distress syndrome; CAD, coronal arterial disease; MI, myocardial infarction; CKD, chronic kidney disease; ESRD, End-stage renal disease; CVA, cerebral vascular accident; TIA, transient ischemic attack; HR, hazard ratio; CI, confidence interval; SD, standard deviation; FIB-4, fibrosis index; AJCC, American Joint Committee on Cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14061398/s1, Figure S1: Kaplan–Meier Survival Curve for 90-Day Mortality for Two Charlson Comorbidity Index score groups; Figure S2: Kaplan–Meier Survival Curve for 90-Day Mortality for Two Charlson Comorbidity Index Score Groups; Figure S3: Kaplan–Meier 5-Year Survival Curve for Long-Term Mortality Rate of Four American Society of Anesthesiologists Physical Status Classification Groups; Figure S4: Kaplan–Meier 5-Year Survival Curve for Long-Term Mortality Rate of Two Charlson Comorbidity Index Score Groups; Table S1: All-Cause 90-Day Mortality Assessment Using the American Society of Anesthesiologists Physical Status Classification Among Patients with Hepatocellular Carcinoma Undergoing Major Hepatectomy; Table S2: All-Cause 90-Day Mortality Assessment Using the Charlson Comorbidity Index Scores Among Patients with Hepatocellular Carcinoma Undergoing Major Hepatectomy.
Author Contributions
Conceptualization, R.-S.S. and S.-Y.W.; methodology, R.-S.S., P.-H.C., Y.-C.C., T.-C.C. and S.-Y.W.; software, B.-C.S. and S.-Y.W.; validation, S.-Y.W.; formal analysis, R.-S.S., Y.-C.C., W.-M.C., M.-F.C., B.-C.S. and S.-Y.W.; investigation, S.-Y.W.; resources, R.-S.S. and S.-Y.W.; data curation, R.-S.S., Y.-C.C. and S.-Y.W.; writing—original draft preparation, R.-S.S.; writing—review and editing, S.-Y.W.; visualization, R.-S.S. and S.-Y.W.; supervision, S.-Y.W.; project administration, B.-C.S. and S.-Y.W.; funding acquisition, R.-S.S. and S.-Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
Lo-Hsu Medical Foundation, LotungPoh-Ai Hospital, supports Szu-Yuan Wus work (Funding Number: 10908, 10909, 11001, 11002, 11003, 11010, 11013, and 11103).
Institutional Review Board Statement
The study protocols were reviewed and approved by the Institutional Review Board of Tzu-Chi Medical Foundation (IRB109-015-B).
Informed Consent Statement
Informed consent was waived because the data sets are covered under the Personal Information Protection Act. We used data from the National Health Insurance Research Database and Taiwan Cancer Registry database. The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. The data used in this study cannot be made available in the article, the Supplementary Files, or in a public repository due to the Personal Information Protection Act executed by Taiwan’s government, starting from 2012. Requests for data can be sent as a formal proposal to obtain approval from the ethics review committee of the appropriate govern-mental department in Taiwan.
Data Availability Statement
The data sets supporting the study conclusions are included in this manuscript and its Supplementary Files.
Conflicts of Interest
The authors have no potential conflicts of interest to declare. The data sets supporting the study conclusions are included in the manuscript.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Llovet J.M., Villanueva A., Marrero J.A., Schwartz M., Meyer T., Galle P.R., Lencioni R., Greten T.F., Kudo M., Mandrekar S.J., et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology. 2021;73((Suppl. 1)):158–191. doi: 10.1002/hep.31327. [DOI] [PubMed] [Google Scholar]
- 2.Kanwal F., Singal A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology. 2019;157:54–64. doi: 10.1053/j.gastro.2019.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seale A.C., Blencowe H., Zaidi A., Ganatra H., Syed S., Engmann C., Newton C.R., Vergnano S., Stoll B.J., Cousens S.N., et al. Neonatal severe bacterial infection impairment estimates in South Asia, sub-Saharan Africa, and Latin America for 2010. Pediatr. Res. 2013;74:73–85. doi: 10.1038/pr.2013.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulik L., El-Serag H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156:477–491.e1. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73:4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y.W., Yong C.C., Lin C.C., Wang C.C., Chen C.L., Cheng Y.F., Wang J.H., Yen Y.H., Chen C.H. Liver resection of hepatocellular carcinoma within and beyond the Barcelona Clinic Liver Cancer guideline recommendations: Results from a high-volume liver surgery center in East Asia. J. Surg. Oncol. 2020;122:1587–1594. doi: 10.1002/jso.26183. [DOI] [PubMed] [Google Scholar]
- 8.Wei A.C., Tung-Ping Poon R., Fan S.T., Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. J. Br. Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 9.Imamura H., Sano K., Sugawara Y., Kokudo N., Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: Decision tree incorporating indocyanine green test. J. Hepato-Biliary-Pancreat. Surg. 2005;12:16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- 10.Wu T., Wang F., Lin Y., Chan K., Yu M., Lee W. Right hepatectomy by the anterior method with liver hanging versus conventional approach for large hepatocellular carcinomas. J. Br. Surg. 2010;97:1070–1078. doi: 10.1002/bjs.7083. [DOI] [PubMed] [Google Scholar]
- 11.Pamecha V., Gurusamy K.S., Sharma D., Davidson B.R. Techniques for liver parenchymal transection: A meta-analysis of randomized controlled trials. HPB. 2009;11:275–281. doi: 10.1111/j.1477-2574.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin H.-M., Lei L.-M., Zhu J., Li G.-L., Min J. Risk factor analysis of perioperative mortality after ruptured bleeding in hepatocellular carcinoma. World J. Gastroenterol. WJG. 2014;20:14921. doi: 10.3748/wjg.v20.i40.14921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrero A., Viganò L., Polastri R., Muratore A., Eminefendic H., Regge D., Capussotti L. Postoperative liver dysfunction and future remnant liver: Where is the limit? World J. Surg. 2007;31:1643–1651. doi: 10.1007/s00268-007-9123-2. [DOI] [PubMed] [Google Scholar]
- 14.Regimbeau J.M., Abdalla E.K., Vauthey J.N., Lauwers G.Y., Durand F., Nagorney D.M., Ikai I., Yamaoka Y., Belghiti J. Risk factors for early death due to recurrence after liver resection for hepatocellular carcinoma: Results of a multicenter study. J. Surg. Oncol. 2004;85:36–41. doi: 10.1002/jso.10284. [DOI] [PubMed] [Google Scholar]
- 15.Friedman L.S. Surgery in the patient with liver disease. Trans. Am. Clin. Clim. Assoc. 2010;121 doi: 10.1016/S0025-7125(16)30851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zacharias T., Ferreira N. Nutritional risk screening 2002 and ASA score predict mortality after elective liver resection for malignancy. Arch. Med. Sci. 2017;13:361. doi: 10.5114/aoms.2017.65273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aronson W.L., McAuliffe M.S., Miller K. Variability in the American Society of Anesthesiologists physical status classification scale. AANA J. 2003;71:265–276. [PubMed] [Google Scholar]
- 18.Fong Y., Brennan M., Cohen A., Heffernan N., Freiman A., Blumgart L. Liver resection in the elderly. J. Br. Surg. 1997;84:1386–1390. [PubMed] [Google Scholar]
- 19.Sundararajan V., Henderson T., Perry C., Muggivan A., Quan H., Ghali W.A. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J. Clin. Epidemiol. 2004;57:1288–1294. doi: 10.1016/j.jclinepi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Hall W.H., Ramachandran R., Narayan S., Jani A.B., Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frenkel W.J., Jongerius E.J., Mandjes-van Uitert M.J., van Munster B.C., de Rooij S.E. Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: A prospective cohort study. J. Am. Geriatr. Soc. 2014;62:342–346. doi: 10.1111/jgs.12635. [DOI] [PubMed] [Google Scholar]
- 22.Laor A., Tal S., Guller V., Zbar A.P., Mavor E. The Charlson Comorbidity Index (CCI) as a mortality predictor after surgery in elderly patients. Am. Surg. 2016;82:22–27. doi: 10.1177/000313481608200113. [DOI] [PubMed] [Google Scholar]
- 23.Qin L., Chen T.-M., Kao Y.-W., Lin K.-C., Yuan K.S.-P., Wu A.T.H., Shia B.-C., Wu S.-Y. Predicting 90-Day Mortality in Locoregionally Advanced Head and Neck Squamous Cell Carcinoma after Curative Surgery. Cancers. 2018;10:392. doi: 10.3390/cancers10100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin K.C., Chen T.M., Yuan K.S., Wu A.T.H., Wu S.Y. Assessment of Predictive Scoring System for 90-Day Mortality Among Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma Who Have Completed Concurrent Chemoradiotherapy. JAMA Netw. Open. 2020;3:e1920671. doi: 10.1001/jamanetworkopen.2019.20671. [DOI] [PubMed] [Google Scholar]
- 25.Su S.Y., Chiang C.J., Yang Y.W., Lee W.C. Secular trends in liver cancer incidence from 1997 to 2014 in Taiwan and projection to 2035: An age-period-cohort analysis. J. Formos. Med. Assoc. 2019;118:444–449. doi: 10.1016/j.jfma.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Chiang C.J., You S.L., Chen C.J., Yang Y.W., Lo W.C., Lai M.S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015;45:291–296. doi: 10.1093/jjco/hyu211. [DOI] [PubMed] [Google Scholar]
- 27.Wen C.P., Tsai S.P., Chung W.S. A 10-year experience with universal health insurance in Taiwan: Measuring changes in health and health disparity. Ann. Intern Med. 2008;148:258–267. doi: 10.7326/0003-4819-148-4-200802190-00004. [DOI] [PubMed] [Google Scholar]
- 28.Wu S.Y., Fang S.C., Hwang O.R., Shih H.J., Shao Y.J. Influence of Baseline Cardiovascular Comorbidities on Mortality after Androgen Deprivation Therapy for Metastatic Prostate Cancer. Cancers. 2020;12:189. doi: 10.3390/cancers12010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shia B.C., Qin L., Lin K.C., Fang C.Y., Tsai L.L., Kao Y.W., Wu S.Y. Outcomes for Elderly Patients Aged 70 to 80 Years or Older with Locally Advanced Oral Cavity Squamous Cell Carcinoma: A Propensity Score-Matched, Nationwide, Oldest Old Patient-Based Cohort Study. Cancers. 2020;12:258. doi: 10.3390/cancers12020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W.C., Liu H.E., Kao Y.W., Qin L., Lin K.C., Fang C.Y., Tsai L.L., Shia B.C., Wu S.Y. Definitive radiotherapy or surgery for early oral squamous cell carcinoma in old and very old patients: A propensity-score-matched, nationwide, population-based cohort study. Radiother. Oncol. 2020;151:214–221. doi: 10.1016/j.radonc.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Yu J.M., Hsieh M.C., Qin L., Zhang J., Wu S.Y. Metformin reduces radiation-induced cardiac toxicity risk in patients having breast cancer. Am. J. Cancer Res. 2019;9:1017–1026. [PMC free article] [PubMed] [Google Scholar]
- 32.Wu S.-Y., Huang E.-Y., Lin H. Optimal Treatments for Cervical Adenocarcinoma. Am. J. Cancer Res. 2019;9:1224. [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy S.K., Barbas A.S., Turley R.S., Steel J.L., Tsung A., Marsh J.W., Geller D.A., Clary B.M. A standard definition of major hepatectomy: Resection of four or more liver segments. HPB. 2011;13:494–502. doi: 10.1111/j.1477-2574.2011.00330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellemgaard A., Luchtenborg M., Iachina M., Jakobsen E., Green A., Krasnik M., Moller H. Role of comorbidity on survival after radiotherapy and chemotherapy for nonsurgically treated lung cancer. J. Thorac. Oncol. 2015;10:272–279. doi: 10.1097/JTO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 35.Sogaard M., Thomsen R.W., Bossen K.S., Sorensen H.T., Norgaard M. The impact of comorbidity on cancer survival: A review. Clin. Epidemiol. 2013;5:3–29. doi: 10.2147/CLEP.S47150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee L., Cheung W.Y., Atkinson E., Krzyzanowska M.K. Impact of comorbidity on chemotherapy use and outcomes in solid tumors: A systematic review. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011;29:106–117. doi: 10.1200/JCO.2010.31.3049. [DOI] [PubMed] [Google Scholar]
- 37.Kuo C.N., Liao Y.M., Kuo L.N., Tsai H.J., Chang W.C., Yen Y. Cancers in Taiwan: Practical insight from epidemiology, treatments, biomarkers, and cost. J. Formos. Med. Assoc. 2020;119:1731–1741. doi: 10.1016/j.jfma.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Zheng R., Qu C., Zhang S., Zeng H., Sun K., Gu X., Xia C., Yang Z., Li H., Wei W., et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res. 2018;30:571–579. doi: 10.21147/j.issn.1000-9604.2018.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poon R.T., Fan S.T., Ng I.O., Wong J. Significance of resection margin in hepatectomy for hepatocellular carcinoma: A critical reappraisal. Ann. Surg. 2000;231:544–551. doi: 10.1097/00000658-200004000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llovet J.M., Kelley R.K., Villanueva A., Singal A.G., Pikarsky E., Roayaie S., Lencioni R., Koike K., Zucman-Rossi J., Finn R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y.Y., Zhao X.H., Ma L., Ye J.Z., Wu F.X., Tang J., You X.M., Xiang B.D., Li L.Q. Comparison of the ability of Child-Pugh score, MELD score, and ICG-R15 to assess preoperative hepatic functional reserve in patients with hepatocellular carcinoma. J. Surg. Oncol. 2018;118:440–445. doi: 10.1002/jso.25184. [DOI] [PubMed] [Google Scholar]
- 42.Feng J.W., Qu Z., Wu B.Q., Sun D.L., Jiang Y. The preoperative fibrosis score 4 predicts posthepatectomy liver failure in patients with hepatocellular carcinoma. Ann. Hepatol. 2019;18:701–707. doi: 10.1016/j.aohep.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Clavien P.-A., Petrowsky H., DeOliveira M.L., Graf R. Strategies for safer liver surgery and partial liver transplantation. N. Engl. J. Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 44.Zhou P., Chen B., Miao X.Y., Zhou J.J., Xiong L., Wen Y., Zou H. Comparison of FIB-4 Index and Child-Pugh Score in Predicting the Outcome of Hepatic Resection for Hepatocellular Carcinoma. J. Gastrointest. Surg. 2020;24:823–831. doi: 10.1007/s11605-019-04123-1. [DOI] [PubMed] [Google Scholar]
- 45.Cieslak K.P., Runge J.H., Heger M., Stoker J., Bennink R.J., Van Gulik T.M. New perspectives in the assessment of future remnant liver. Dig. Surg. 2014;31:255–268. doi: 10.1159/000364836. [DOI] [PubMed] [Google Scholar]
- 46.Petrowsky H., Fritsch R., Guckenberger M., De Oliveira M.L., Dutkowski P., Clavien P.-A. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 2020;17:755–772. doi: 10.1038/s41575-020-0314-8. [DOI] [PubMed] [Google Scholar]
- 47.Zaydfudim V.M., Turrentine F.E., Smolkin M.E., Bauer T.B., Adams R.B., McMurry T.L. The impact of cirrhosis and MELD score on postoperative morbidity and mortality among patients selected for liver resection. Am. J. Surg. 2020;220:682–686. doi: 10.1016/j.amjsurg.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahnemai-Azar A.A., Cloyd J.M., Weber S.M., Dillhoff M., Schmidt C., Winslow E.R., Pawlik T.M. Update on Liver Failure Following Hepatic Resection: Strategies for Prediction and Avoidance of Post-operative Liver Insufficiency. J. Clin. Transl. Hepatol. 2018;6:97–104. doi: 10.14218/JCTH.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Y.-Y., Xiang B.-D., Ma L., Zhong J.-H., Ye J.-Z., Wang K., Xing B.-C., Li L.-Q. Development and validation of a nomogram to preoperatively estimate post-hepatectomy liver dysfunction risk and long-term survival in patients with hepatocellular carcinoma. Ann. Surg. 2021;274:e1209–e1217. doi: 10.1097/SLA.0000000000003803. [DOI] [PubMed] [Google Scholar]
- 50.Yoshikawa T., Nomi T., Hokuto D., Kamitani N., Matsuo Y., Sho M. Outcomes in Patients with Chronic Kidney Disease After Liver Resection for Hepatocellular Carcinoma. World J. Surg. 2021;45:598–606. doi: 10.1007/s00268-020-05829-z. [DOI] [PubMed] [Google Scholar]
- 51.Bolder U., Brune A., Schmidt S., Tacke J., Jauch K.W., Lohlein D. Preoperative assessment of mortality risk in hepatic resection by clinical variables: A multivariate analysis. Liver Transpl. Surg. 1999;5:227–237. doi: 10.1002/lt.500050302. [DOI] [PubMed] [Google Scholar]
- 52.Meyer S. Grading of patients for surgical procedures. Anesthesiology. 1941;2:281–284. [Google Scholar]
- 53.Daabiss M. American Society of Anaesthesiologists physical status classification. Indian J. Anaesth. 2011;55:111–115. doi: 10.4103/0019-5049.79879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kirkland L.L., Kashiwagi D.T., Burton M.C., Cha S., Varkey P. The Charlson Comorbidity Index Score as a predictor of 30-day mortality after hip fracture surgery. Am. J. Med. Qual. 2011;26:461–467. doi: 10.1177/1062860611402188. [DOI] [PubMed] [Google Scholar]
- 55.Volk M.L., Hernandez J.C., Lok A.S., Marrero J.A. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transplant. 2007;13:1515–1520. doi: 10.1002/lt.21172. [DOI] [PubMed] [Google Scholar]
- 56.Hsieh C.Y., Su C.C., Shao S.C., Sung S.F., Lin S.J., Kao Yang Y.H., Lai E.C. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019;11:349–358. doi: 10.2147/CLEP.S196293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu S.-Y., Chang C.-L., Chen C.-I., Huang C.-C. Comparison of Acute and Chronic Surgical Complications Following Robot-Assisted, Laparoscopic, and Traditional Open Radical Prostatectomy Among Men in Taiwan. JAMA Netw. Open. 2021;4:e2120156. doi: 10.1001/jamanetworkopen.2021.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kao W.H., Hong J.H., See L.C., Yu H.P., Hsu J.T., Chou I.J., Chou W.C., Chiou M.J., Wang C.C., Kuo C.F. Validity of cancer diagnosis in the National Health Insurance database compared with the linked National Cancer Registry in Taiwan. Pharmacoepidemiol. Drug Saf. 2018;27:1060–1066. doi: 10.1002/pds.4267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets supporting the study conclusions are included in this manuscript and its Supplementary Files.