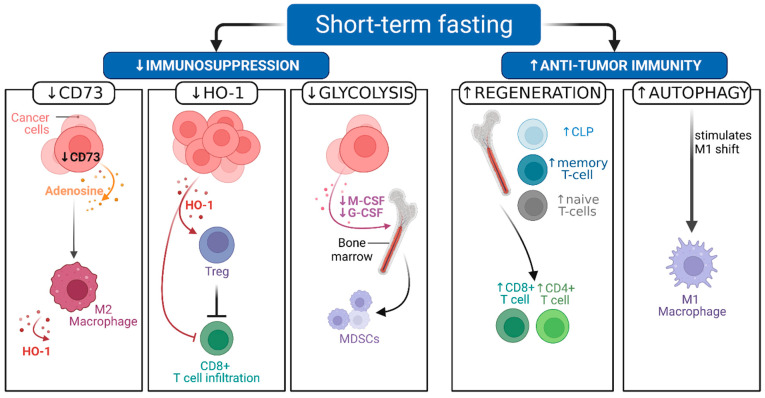
Figure 2.
Immunomodulatory mechanisms of short-term fasting [STF] during anticancer therapy. STF reduces immunosuppression and enhances antitumor immunity via the following mechanisms established from preclinical studies: CD73 downregulation in cancer cells causes decreased adenosine release, which in turn diminishes immunosuppressive M2-type macrophage polarization. Decreased heme oxygenase-1 (HO-1) production by cancer cells (and M2 macrophages) releases inhibition of regulatory T (Tregs) cells on CD8+ cytotoxic T cells directly as well as direct inhibition from HO-1. Lowered glycolysis inhibits macrophage and granulocyte colony-stimulating factor (M-CSF, G-CSF) secretion by cancer cells. Consequently less myeloid derived suppressor cells are mobilized from the bone marrow. Hematopoietic stem cell regeneration of common lymphoid progenitors (CLP), naïve T cells and accumulation of memory T cells is observed centrally. Peripheral increase of CD8+ and CD4+ T cells is observed after refeeding and might replenish exhausted T cells as well as increase tumor antigen recognition chance. Autophagy induction stimulates tumoricidal M1 macrophage differentiation, which can support antitumor immunity. Figure 2 is adapted from “Cancer Immunoediting”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates.