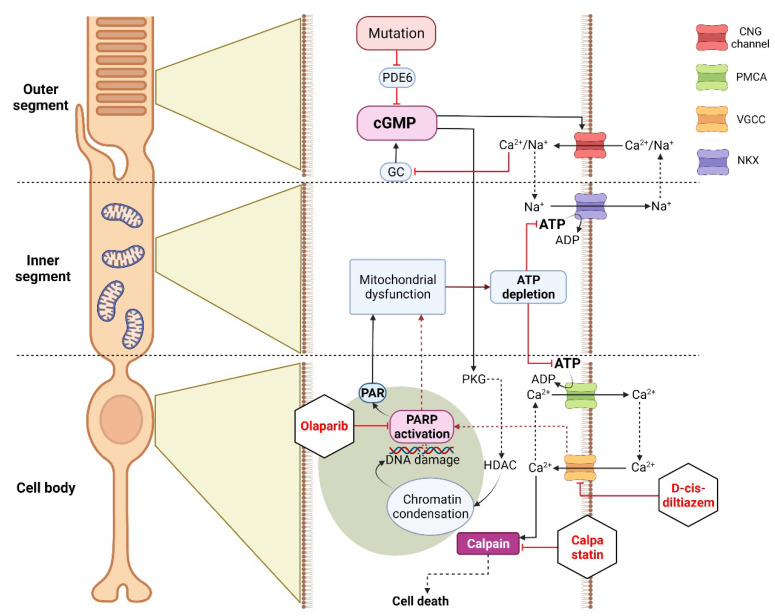
Figure 7.
Differential effects of experimental conditions on cGMP-dependent cell death in rd1 photoreceptors. The mutation-induced cGMP accumulation activates cyclic nucleotide-gated (CNG) channels in the outer segment, leading to Na+-and Ca2+-influx and photoreceptor depolarization. This leads to opening of voltage-gated Ca2+-channels (VGCCs) in the cell body, causing further Ca2+- influx. In the cell body, high Ca2+ levels may activate calpain if not controlled by ATP-dependent plasma membrane Ca2+-ATPase (PMCA). In addition, cGMP-dependent activation of protein kinase G (PKG) has been associated with histone-deacetylase (HDAC) activity, causing chromatin condensation and DNA breaks, which may trigger PARP activation. Excessive consumption of NAD+ by PARP and the production of PAR may cause mitochondrial dysfunction, leading to ATP shortage. Calpastatin treatment blocks calpain activation, decreasing proteolytic damage to the cell, even in the presence of CNG channel/VGCC-mediated Ca2+-influx. D-cis-diltiazem inhibits VGCCs in the cell body, reducing intracellular Ca2+-levels and calpain activity. Moreover, VGCCs could be involved in PARP activation, even though D-cis-diltiazem fails to delay rd1 rod degeneration. Olaparib blocks PARP activity, decreasing NAD+ consumption and PAR generation. This may preserve mitochondrial function and intracellular ATP levels, allowing PMCA to extrude Ca2+ and keeping calpain activity low.