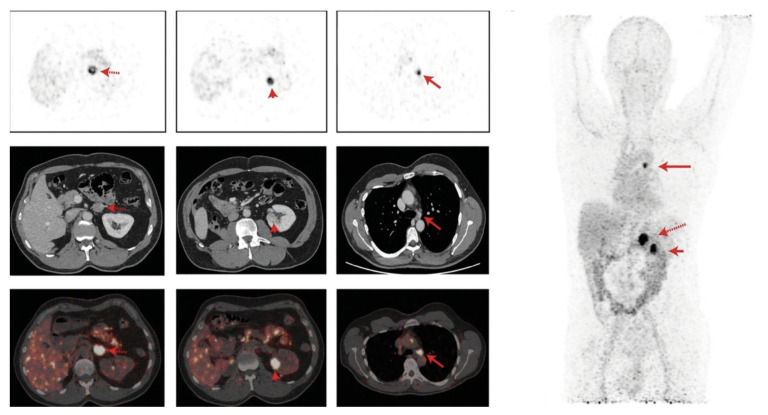
Figure 2.
89Zr-girentuximab in renal cancer patient. Left panel: top row, axial PET imaging at 168 h post administration (h p.a).; middle row, contrast enhanced CT; bottom row, fused PET/CT imaging showing (from left to right) 89Zr-girentuximab uptake in the adrenal gland (red arrow), left kidney (red arrowhead), and mediastinal lymph node (red arrow). Right panel: the maximum intensity projection (MIP) whole-body PET image at 168 h p.a. Reprinted with permission from Merkx, R.I.J.; Lobeek, D.; Konijnenberg, M.; et al. Phase I study to assess safety, biodistribution, and radiation dosimetry for 89Zr-girentuximab in patients with renal cell carcinoma. Eur J Nucl Med Mol Imaging, 2021. 48, 3277–3285 [43].