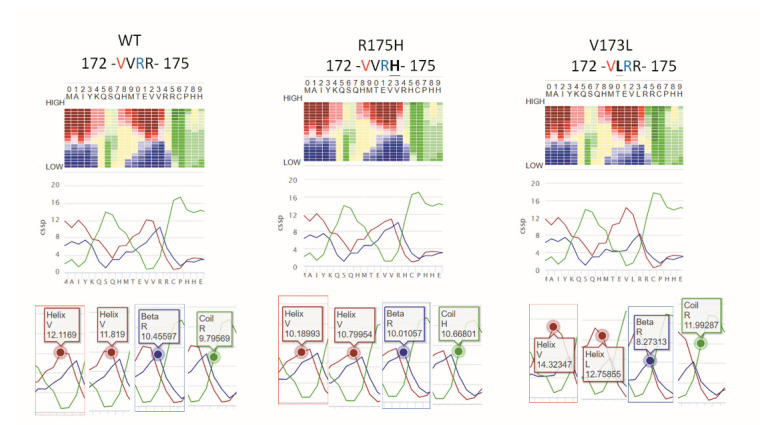
Figure 3.
The CSSP value of the β-strand of flanking amino acids (a.a.) on p53 V173L is lower than the wild-type (WT) and R175H. The propensity for each of the three secondary structure elements, α helix (red), β-strand (blue) and coil (green), is calculated at 20 different levels, so the sum of the CSSP scores is 20. R175H flanking a.a. reduces α-strand CSSP from 12.1169 (WT) to 10.18993. V173L flanking a.a. reduces β-strand CSSP from 10.45597 (WT) to 8.27313. V173L flanking a.a. has a 14.32347 α-strand CSSP larger than WT (12.1169) and R175H (10.18993).