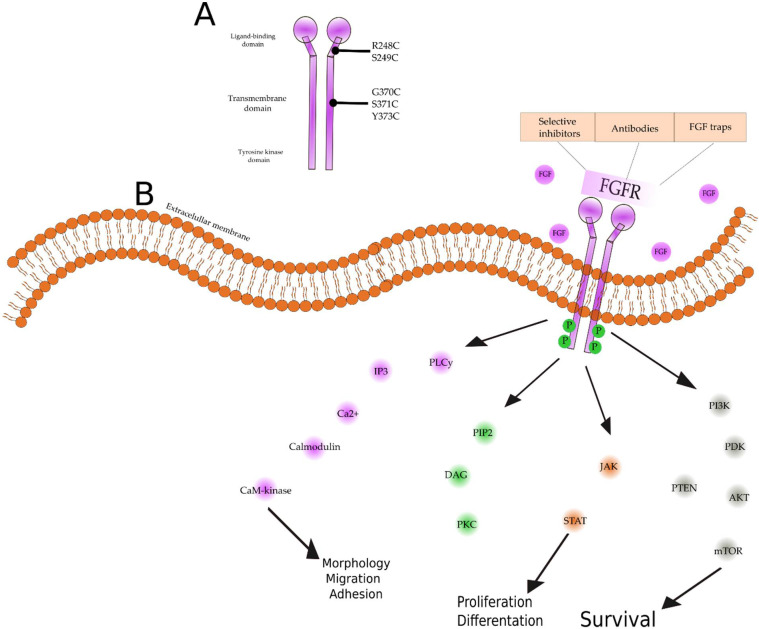
Figure 1.
(A) Structure of fibroblast growth factor receptor and main mutations found in advanced bladder cancer. FGFRs are tyrosine kinase receptors, consisting of a heparin-binding sequence and immunoglobulin-like extracellular sequence and a hydrophobic transmembrane domain and intracellular tyrosine kinase domain [37]. Most common genetic changes are missense mutations and FGFR3-TACC3 fusion, primarily in ligand-binding domains (R248C and S249C), less frequently in the transmembrane domain (G370C, S371C and Y373C), rarely in the tyrosine kinase domain [27,37]. Alterations lead to overexpression and hyperactivation of FGFR. (B) The FGF signaling pathway. Ligand binds to an FGFR monomer, which leads to dimerization and intracellular phosphorylation, resulting in conformational changes. This provides the means to start signaling pathways for FGFRs. Activated FGFRs phosphorylate FRS2, which opens the way for PI3K, AKT, mTOR, or the RAS/RAF/MEK/MAPK cascade. Activated FGFRs also phosphorylate JAK kinases, which lead to STAT activation. FGFRs can also recruit and phosphorylate PLCγ, thereby initiating signaling through the DAG/PKC or IP3-Ca2+. All of those pathways have a crucial role in tumor development [25,27,38,39,40]. FGFRs (fibroblast growth factor receptors), FRS2 (fibroblast growth factor receptor substrate 2), PI3K (phosphoinositide 3-kinase), AKT (protein kinase B), mTOR (mammalian target of rapamycin), MAPK (mitogen-activated protein kinase), JAK (Janus kinase), STAT (signal transducer and activator of transcription), PLCγ (phospholipase C gamma), DAG (dystroglycan), PKC (protein kinase C), IP3 (inositol trisphosphate).