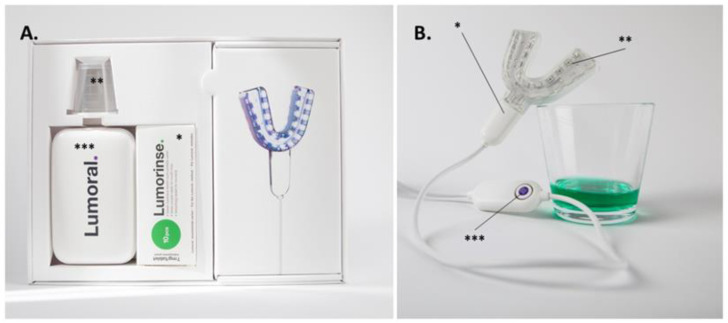
Figure 1.
The dual-light aPDT device was used in the study. (A) The packaging provided for the study subjects included the effervescent ICG tablets (*) to be dissolved in 30 mL of water, for which a measuring cup (**) was provided. A power source (***) was provided for the mouthguard-type light applicator (B). The mouthpiece (*) is composed of 48 LED components (**) able to provide 405 nm and 810 nm light simultaneously. Symmetrically assembled LEDs provided light for both the maxillary and mandibular dental arches. The button on the control unit provided a treatment time of 10 min (***). Dissolved mouth rinse can be seen in the glass.