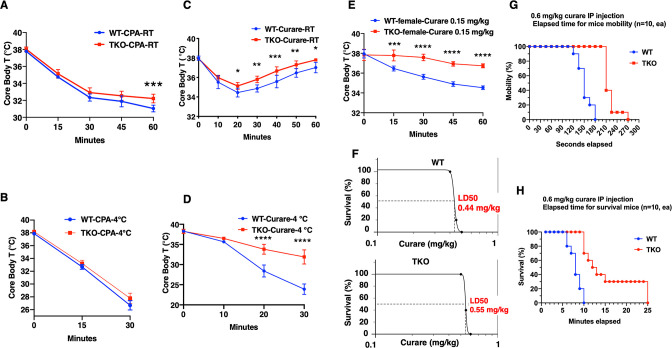
Figure 2. TIGAR knockout mice are resistant to tubocurare but not cyclopiazonic acid (CPA).
(A) Wild type (WT) and whole-body Tigar knockout (TKO) male mice (n = 6) were intraperitoneally injected with CPA (10 mg/kg body weight), and then core body temperature was measured every 15 min at room temperature. (B) WT and TKO male mice (n = 6) were intraperitoneally injected with CPA (10 mg/kg body weight) at room temperature, and then shifted to 4°C for 30 min, with core body temperature measured every 15 min. (C) WT and TKO male mice (n = 6) were intraperitoneally injected with tubocurare (0.4 mg/kg body weight), and then core body temperature was measured every 10 minutes at room temperature. (D) WT and TKO male mice (n = 6) were intraperitoneally injected with tubocurare (0.4 mg/kg body weight) at room temperature, and then 10 min later shifted to 4°C for 30 min, with core body temperature measured every 10 min. (E) WT and TKO female mice (n = 6) were intraperitoneally injected with tubocurare (0.15 mg/kg body weight), and then core body temperature was measured every 15 min at room temperature. (F) WT and TKO male mice (n = 10) were intraperitoneally injected with different tubocurare doses, and the LD50 of curare was calculated using an online software LD50 Calculator (AAT Bioquest, Inc, Sunnyvale, CA). (G) WT and TKO male mice (n = 10) were intraperitoneally injected with a lethal tubocurare dose (0.6 mg/kg body weight), and the number of mice undergoing complete paralysis was plotted as a function of time in seconds. (H) The time to death (absence of respiration) of the same mice was plotted as a function of time in minutes. Statistical analyses are described in ‘Method details,’ and the data are presented as the mean ± SD. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.