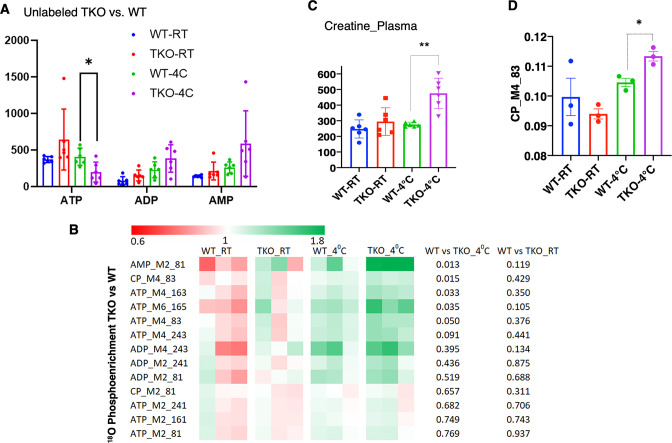
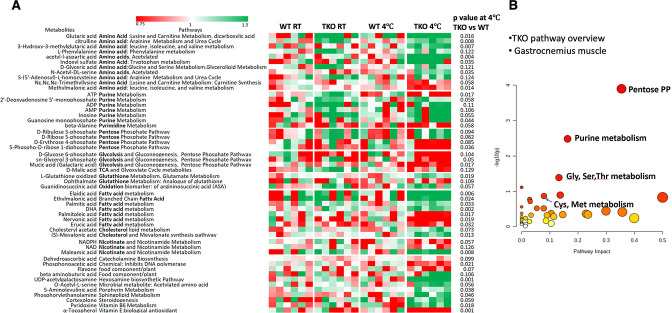
Figure 3. TIGAR-deficient mice display increased ATP turnover in skeletal muscle.
(A) Wild type (WT) and whole-body Tigar knockout (TKO) male mice (n = 6) were maintained at room temperature (RT) or shifted to 4°C for 1 hr. Quadricep white skeletal muscles were isolated, and extracts assayed for ATP, ADP, and AMP levels as described in ‘Method details.’ (B) WT and TKO male mice (n = 3–4 per condition) were kept at RT or shifted to 4°C for 1 hr and then given an oral gavage of 0.3 ml pure H2O18 for 10 min at 4°C and an intraperitoneal (IP) injection of 1 ml pure H2O18 for another 10 min at 4°C. The quadricep muscles were freeze-clamped with liquid N2 and extracts prepared for mass spectroscopic analyses. The heatmap shows the O18 enrichment fraction of ATP, ADP, AMP, and creatine-phosphate. (C) WT and TKO male mice (n = 6) were maintained at RT or shifted to 4°C for 1 hr, and plasma creatine levels were determined by metabolomics analyses. (D) The CP_M4_83 data was generated from the same experimental setting as (B), indicating the increase in phosphocreatine turnover in TKO compared to that in WT at 4°C. Statistical analyses are described in ‘Method details,’ and the data are presented as the mean ± SD. *p<0.05, ***p<0.01.