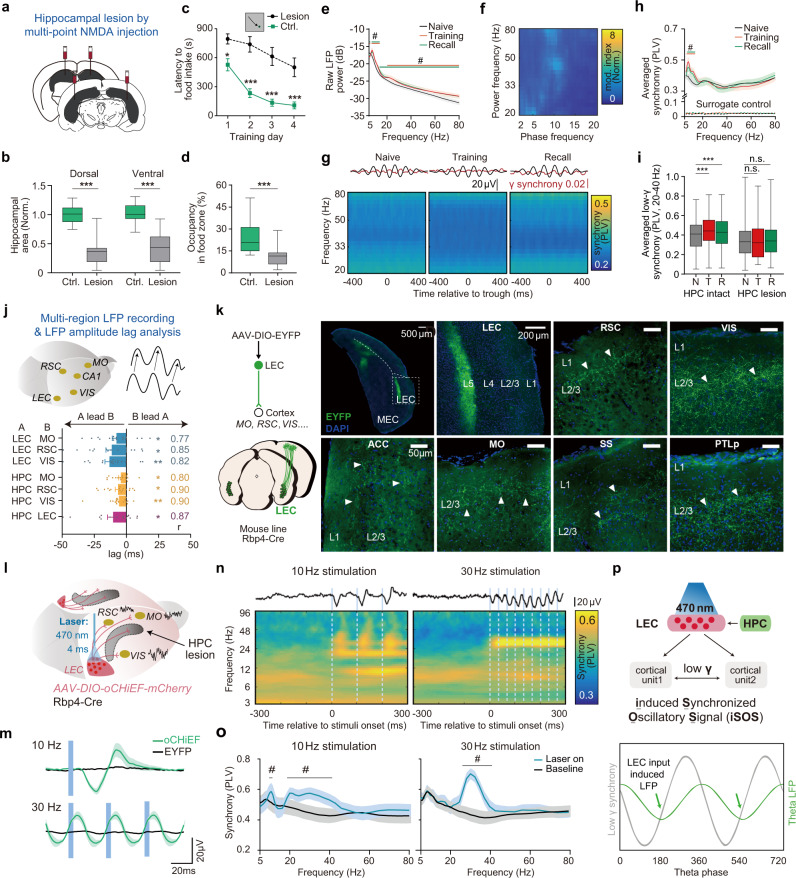
Fig. 2. HPC-dependent long-range cortical gamma synchrony is regulated by layer 5 LEC cortical projects and restored by iSOS in HPC-lesioned mice.
a, b Effective bilateral hippocampal lesion in mice by multipoint NMDA injection. a Experimental scheme. b Quantification of the hippocampal residue size. (NControl mice = 14; NHPC-lesioned = 15; Dorsal: two-sided t-test, t(27) = 7.9; P < 0.0001; Ventral, two-sided t-test, t(27) = 6.8, P < 0.0001). c Learning curves show impairment of HPC-lesioned mice in the spatial memory task (NControl mice = 24; NHPC-lesioned = 15; ANOVA, Time factor: F(3, 148) = 12.61, P < 0.0001. Group factor: F(1, 148) = 87.7, P < 0.0001; Interaction, F(3, 148) = 1.484, P = 0.2213; Bonferroni post-hoc test, PDay1 = 0.0111, PDay2 < 0.0001, PDay3 < 0.0001, PDay4 < 0.0001). d HPC-lesioned mice did not prefer the food zone in the probe trials (same mice number as (c), two-sided t-test, t(37) = 4.1, P = 0.0002). e, f Theta and gamma power increased in training and recall trials while gamma power was no longer coupled to cortical theta phase in HPC-lesioned mice (35 electrodes from 12 mice, NMO = 11, NRSC = 12, NVIS = 12). Here training shows the data from the first day of training, same as the following graphs. See results for each region for each day in the supplementary Fig. 2c. e Averaged raw cortical LFP power from three regions of different trials of HPC-lesioned mice (q < 0.05, FDR corrected, significant frequencies were noted on the graph). f Phase-power modulation index comodulograms of training of HPC-lesioned mice, averaged from all regions. g, h Neither the coupling between gamma synchrony and cortical theta phase (Fig. 1g) nor the elevation of long-range cortical synchrony (Fig. 1h) can be detected during training and memory recall in HPC-lesioned mice (34 electrode pairs from 12 mice. NMO-RSC = 11, NRSC-VIS = 12, NMO-VIS = 11). g Cortical gamma synchrony no longer coupled to the cortical theta phase in HPC lesion mice. Top, averaged theta wave (black) and 30 Hz cortical synchrony (red). Bottom, averaged phase(theta)-synchrony(gamma) spectrogram from all pairs. h Comparison of averaged overall synchrony in three kinds of trials (q < 0.05, FDR corrected, significant frequencies were noted on the graph. See raw power, synchrony, comodulograms of each regions for each day and corresponding quantification in Supplementary Figs. 2–3). i Comparison of the overall low gamma synchrony between HPC intact mice and HPC lesion mice across the training process. N, naive; T, training day1; R, recall. (same data as Fig. 1g–h and Fig. 2g–h. Two-way ANOVA, FInteraction (2,208) = 9.56, P < 0.0001, FLesion vs. Intact (1,204) = 3.406, P = 0.0678; FNaive vs. Training vs. Recall (2,208) = 5.705, P < 0.0001. Bonferroni post-hoc test, HPC intact: PTraining vs. Naive < 0.0001, PRecall vs. Naive < 0.0001). j Top, multi-region LFP recording and diagram for theta oscillation amplitude-based cross-correlation analysis. Bottom, dHPC theta lead LEC and LEC theta lead theta of cortical regions in training trials (training day1). Correlation coefficients of the max lag are noted on the right. (Wilcoxon signed-rank test, two-sided, compared to 0. NLEC-MO = 18, NLEC-RSC = 18, NLEC-VIS = 18, NdHPC-MO = 17, NdHPC-RSC = 17, NdHPC-VIS = 17, NdHPC-LEC = 16 from 19 mice). See lag summary of other learning state in Supplementary Fig. 6. k Efferent axons from L5 neurons of LEC were detected in a wide range of cortical areas in L2/3, including, MO, RSC, VIS, SS, PTLp (Posterior parietal cortex) and ACC (Anterior cingulate cortex). Axons were labeled by EYFP via virus (AAV2/9-DIO-EYFP) injection in LEC of Rbp4Cre mice. Scale bar, 50 μm. (three mice brain were sectioned and show similar results, represnetive images here are from one of them). l–m Artificial co-activation of LEC axons induced long-range cortical gamma synchrony. To detect the pure effect of optogenetic stimulation, cell body activation was done in homecage using awake HPC-lesioned mice to minimize the task-engaged cortical synchrony. l 470 nm laser-activated neurons expressing oCHiEF-mCherry in LEC (4 ms per pulse) induced synchronization oscillatory signals (iSOS) of LFP in multiple cortical areas simultaneously. m Blue light stimulation at either 10 Hz or 30 Hz in LEC could induce LFP responses simultaneously in MO, VIS and RSC in oCHiEF-expressing mice (green lines) but not EYFP expressing mice (oCHiEF group: n = 21 electrodes, including electrodes in RSC, MO, and VIS, EYFP group: n = 14 electrodes, including electrodes in RSC, MO, and VIS, lines are the averaged evoke potential of RSC, MO, and VIS). The evoked potential period (~36 ms, gamma frequency, ~30 Hz) did not change across stimuli frequencies. See surface axonal stimuli in Supplementary Fig. 9a–b. n Averaged cortical synchrony heatmap before and after stimulation. Top black lines, averaged LFP traces for each stimulation. Blue lines, laser stimuli. The spectrogram is the average of all cortical pairs including, RSC-MO, RSC-VIS, and VIS-MO. o Quantification of panel n. Baseline, averaged PLV before laser stimulation. Laser on, averaged PLV at 10–50 ms after each pulse, significant frequency ranges (lines above) lie at gamma band (q < 0.05, FDR corrected, 16 electrode pairs). p Illustration summarizes that long-range gamma synchrony is mediated by HPC-LEC and coupled to the theta-rhythm during memory encoding and retrieval (top). Endogenous synchronized LEC axonal activation may induce cortical synchronization oscillatory signals (iSOS), which is phase lock to theta phase, during memory encoding and retrieval (bottom). Shadow of lines and error bar shows lines show S.E.M. ***P < 0.001, #q < 0.05. For all box plot, whiskers show min and max, box shows 25th, median and 75th percentile.