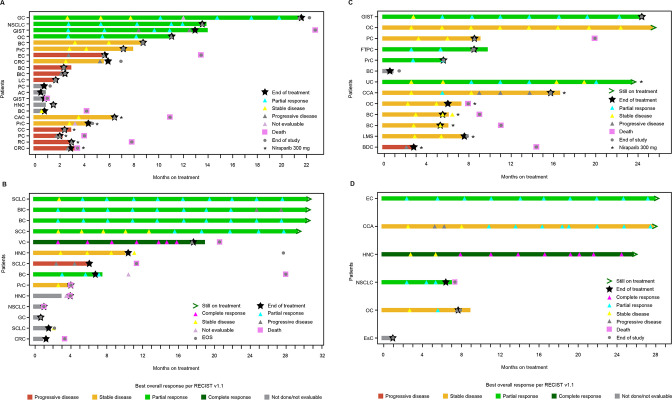
Figure 2.
Duration of response and treatment in part A (A), part B (B), part C (C), and part D (D). AC, appendix cancer; BC, breast cancer; BDC, bile duct cancer; BLC, bladder cancer; CAC, colon adenocarcinoma; CC, colon cancer; CCA, cholangiocarcinoma; CRC, colorectal cancer; EC, endometrial cancer; EOS, end of study; ESC, esophageal cancer; FTPC, fallopian tube papillary carcinoma; GC, gastrointestinal cancer; GIST, gastrointestinal stromal tumor; HNC, head and neck cancer; LC, liver cancer; LMS, leiomyosarcoma; NSCLC, non-small cell lung carcinoma; OC, ovarian cancer; PC, pancreatic cancer; PRC, prostate cancer; RC, rectal cancer; RECIST, Response Evaluation Criteria in Solid Tumors; SCC, squamous cell carcinoma; SCLC, small cell lung cancer; UC, uterine cancer; VC, vulvar cancer.