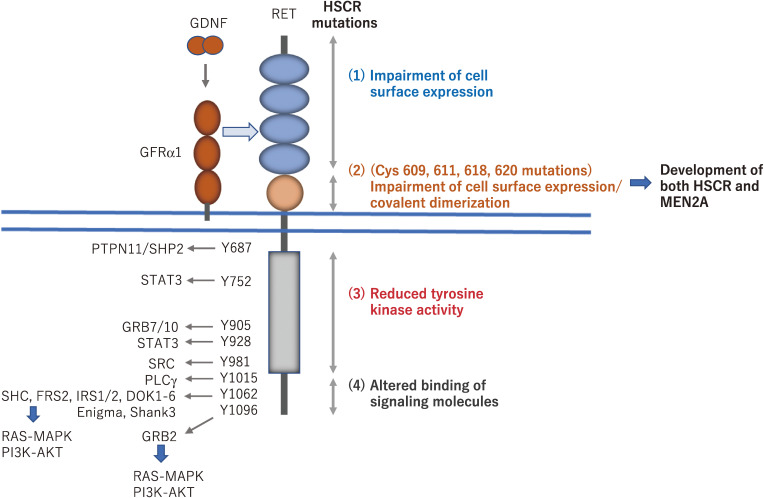
Figure 2.
Intracellular signaling pathways activated by RET via phosphotyrosines and RET mutations in Hirschsprung’s disease (HSCR). Phosphorylated tyrosines in the intracellular domain of RET interact with a wide range of adaptor proteins, which leads to the activation of downstream signaling pathways, including the RAS/MAPK and PI3K-AKT pathways. For example, phosphotyrosine 1062 represents a multifunctional docking site for SHC, FRS2, and DOK family proteins. Missense mutations identified in HSCR patients are distributed along the whole coding sequence of the RET gene. Based on functional analyses of mutant RET, the HSCR mutations are classified as follows. (1) Most mutations in the RET extracellular domain impair its cell surface expression, most likely due to misfolding of the RET protein. (2) Mutations of Cys609, 611, 618, and 620 can result in the development of both HSCR (loss-of-function) and multiple endocrine neoplasia type 2A (MEN2A) (gain-of-function) phenotypes that are caused by impaired cell surface expression of RET in ENCDCs and covalent dimerization in thyroid C cells, respectively. (3) Mutations in the tyrosine kinase domain almost completely or partially disturb the RET kinase activity, resulting in the impairment of RAS/MAPK, PI3K/AKT, and/or PLCγ signaling pathways. (4) Mutations in the carboxyl-terminal tail alter the binding of adaptor proteins such as SHC.