FIG. 2.
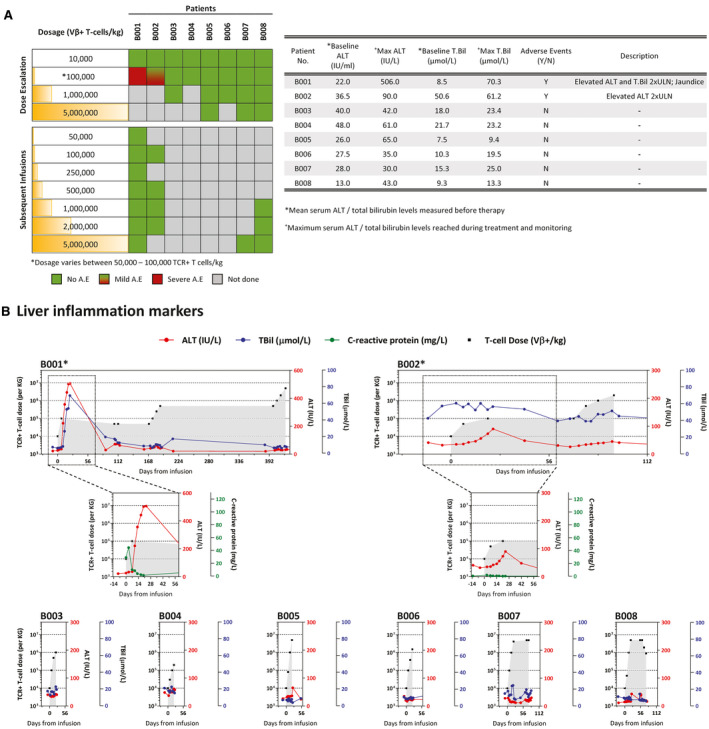
HBV‐specific TCR T‐cell therapy can cause a self‐limiting and reversible liver‐specific adverse event in some patients. (A) Occurrence of adverse events in relation to the dose of HBV‐specific TCR T cells infused into the patient (left). Table summarizes the adverse events that occurred during the course of the therapy, and the liver‐specific parameters recorded at baseline and the maximum levels achieved during treatment. (B) Longitudinal levels of liver inflammation markers ALT (red) and TBil (blue) of all patients treated with HBV‐specific TCR T cells. CRP levels (green) of patients with documented adverse events are shown in the respective inserts. The number of HBV‐specific TCR T cells infused are indicated in gray. *Patients with reported adverse events.
