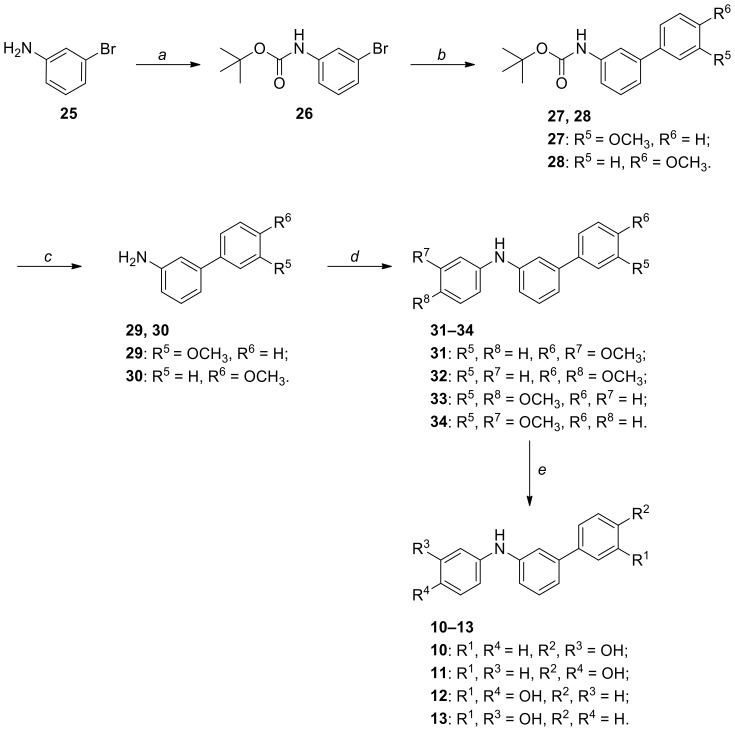
Scheme 2.
Synthesis of diarylamine derivatives 10–13. Reagents and conditions: (a) (Boc)2O, Et3N, anhydrous THF, 0 °C to RT, 24 h [62%]; (b) 3- or 4-methoxybenzeneboronic acid, Pd(OAc)2, PPh3, 2 M aq. Na2CO3, EtOH, toluene, 100 °C, 18 h [89%]; (c) TFA, anhydrous CH2Cl2, 0 °C to RT, 2 h [85–94%]; (d) 3- or 4-bromoanisole, Pd2dba3, XPhos, K3PO4, anhydrous toluene, 100 °C, 20 h [39–91%]; (e) 1 M BBr3 in CH2Cl2, anhydrous CH2Cl2, −15 to 0 °C, then RT, 3 h [72–98%].