Table 1. Optimization of the Alkylation–Annulation Reactiona.
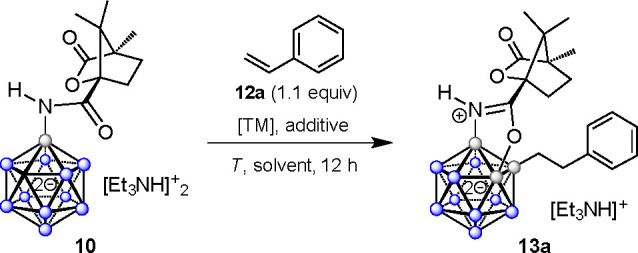
| no. | [TM]b (amt, mol %) | additive (amt, equiv)c | T (°C) | solvent | yield (%) |
|---|---|---|---|---|---|
| 1 | [Rh] (10) | 60 | MeCN | ||
| 2 | [Ir] (10) | 60 | MeCN | ||
| 3 | [Rh] (10) | [Cu] (2) | 60 | MeCN | 50 |
| 4 | [Rh] (10) | [Ag] (1) | 60 | MeCN | |
| 5 | [Ir] (10) | [Cu] (2) | 60 | MeCN | 50 |
| 6 | [Ir] (10) | [Ag] (1) | 60 | MeCN | |
| 7 | [Ir] (10) | [Cu] (2) | 40 | MeCN | 65 |
| 8 | [Rh] (10) | [Cu] (2) | 40 | MeCN | 75 |
| 9 | [Rh] (5) | [Cu] (2) | 25 | MeCN | 82 |
| 10 | [Ir] (5) | [Cu] (2) | 25 | MeCN | 72 |
| 11 | [Rh] (2.5) | [Cu] (2) | 25 | MeCN | 85 |
| 12 | [Rh] (2.5) | [Cu] (2) | 25 | EtOH | |
| 13 | [Rh] (2.5) | [Cu] (2) | 25 | Acetone | 52 |
| 14 | [Rh] (2.5) | [Cu] (2) | 25 | MeOH | |
| 15 | [Rh] (2.5) | [Cu] (2) | 25 | THF | 56 |
| 16 | [Rh] (2.5) | [Cu] (2) | 25 | DCE | 48 |
Reactions were conducted on a 20 mg scale in 1 mL of the solvent in a glass vial sealed with a screw cap.
Definitions: [Rh], [RhCp*Cl2]2; [Ir] = [IrCp*Cl2]2.
Definitions: [Cu], Cu(OAc)2·H2O; [Ag] = AgOAc.
