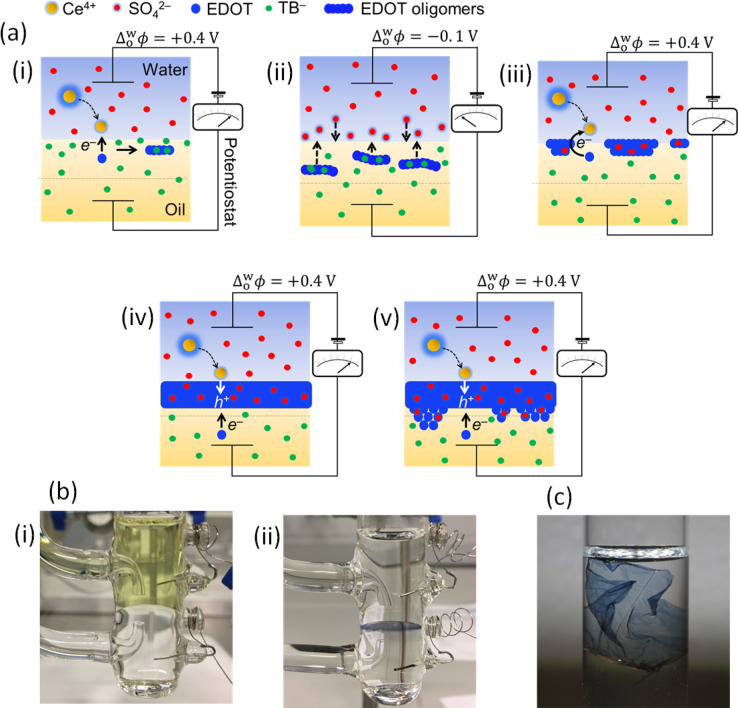
Figure 1.
The mechanism of PEDOT interfacial electrosynthesis at the interface between two immiscible electrolyte solutions (ITIES). (a) The mechanism is schematically shown as five distinct steps with time: (i) interfacial electron transfer (IET) at a positive externally applied interfacial Galvani potential difference (Δowϕ = +0.4 V) between the aqueous Ce4+ oxidant and organic EDOT monomer to form cationic EDOT oligomers, (ii) interfacial adsorption of the cationic EDOT oligomers at a more negative Δoϕ (−0.1 V) through an ion-pairing and interchange interaction with the aqueous SO42 – anions, (iii) autocatalytic IET between Ce4+ and EDOT at adsorbed EDOT oligomer sites that act as interfacial bipolar electrodes, (iv) adsorbed EDOT oligomer coalescence to form a highly compact 2D PEDOT thin film at the ITIES that is flat on both sides and heavily doped with aqueous SO42– anions, and (v) continued IET leading to a secondary growth process into the organic phase and the formation of a porous 3D structure on the organic-facing side as the thickness of the PEDOT thin film increases. (b) Four-electrode electrochemical cell (i) before and (ii) after interfacial electrosynthesis. The acidic aqueous phase, containing the yellow Ce4+ oxidant, is on top and the more dense α, α, α-trifluorotoluene (TFT) organic solution containing the EDOT monomer is on the bottom. PEDOT forms exclusively as a thin blue film at the polarized liquid|liquid (L|L) interface. (c) A PEDOT thin film removed from a large ITIES and stored in an acetone/0.2 M H2SO4 mixture to minimize gradual undoping.