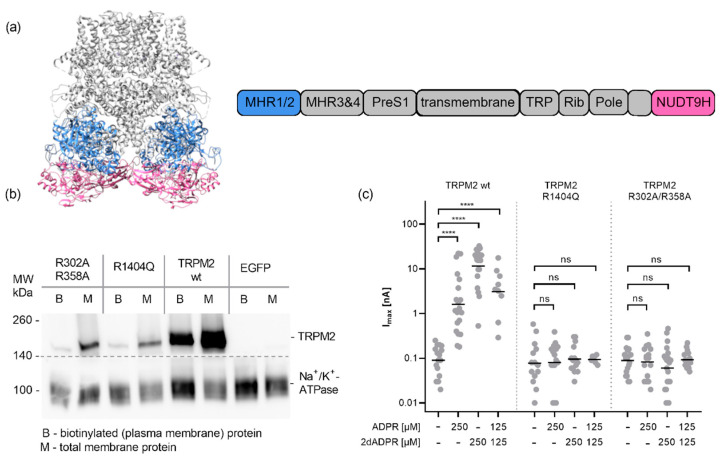
Figure 3.
Effect of mutations in the MHR1/2 domain and in the NUDT9H domain on activation of TRPM2 by ADPR and 2′-deoxy-ADPR. (a) Structure of TRPM2, visualization of PDB:6PUS [18] generated in UCSF chimera [34], MHR1/2 (blue) and NUDT9H (magenta) domains are highlighted. (b) Effect of mutations on the plasma membrane localisation of TRPM2. Transiently transfected HEK293 cells (as above, additionally, empty pIRES-EGFP vector control was used) were treated with a nonmembrane permeant biotinylating agent. Cells were harvested and total membrane proteins isolated (M). The biotinylated proteins were enriched using neutravidin agarose beads. After separation on a 4–15% SDS-PAGE proteins were transferred to a PVDF membrane and detected by chemoluminescence using primary antibodies against human TRPM2 (upper part, NB 500-241, Novus Biologicals) and human Na+/K+-ATPase ((lower part, #3010, Cell Signalling Technology) and an HRP-conjugated secondary antibody, both parts, Dianova #111-035-045) (c) HEK293 cells were either transiently transfected with expression vectors for human TRPM2 with mutations in either the NUDT9H domain (R1404Q) or in the MHR1/2 domain (R30/R358A) or the wild type channel. 24 h post-transfection cells were placed in a bath solution with NMDG and infused via the patch pipette with an intracellular solution buffered to a Ca2+ of 200 nM with EGTA and containing either ADPR, 2′-deoxy-ADPR or a combination of both. Max currents at +15 mV obtained from repetitive voltage ramps are shown on a log scale. Log-transformed data were tested against the respective control by one-way ANOVA followed by a post hoc t test with Bonferroni correction; the mean is indicated by a horizontal bar (**** p < 0.0001, ns not significant).