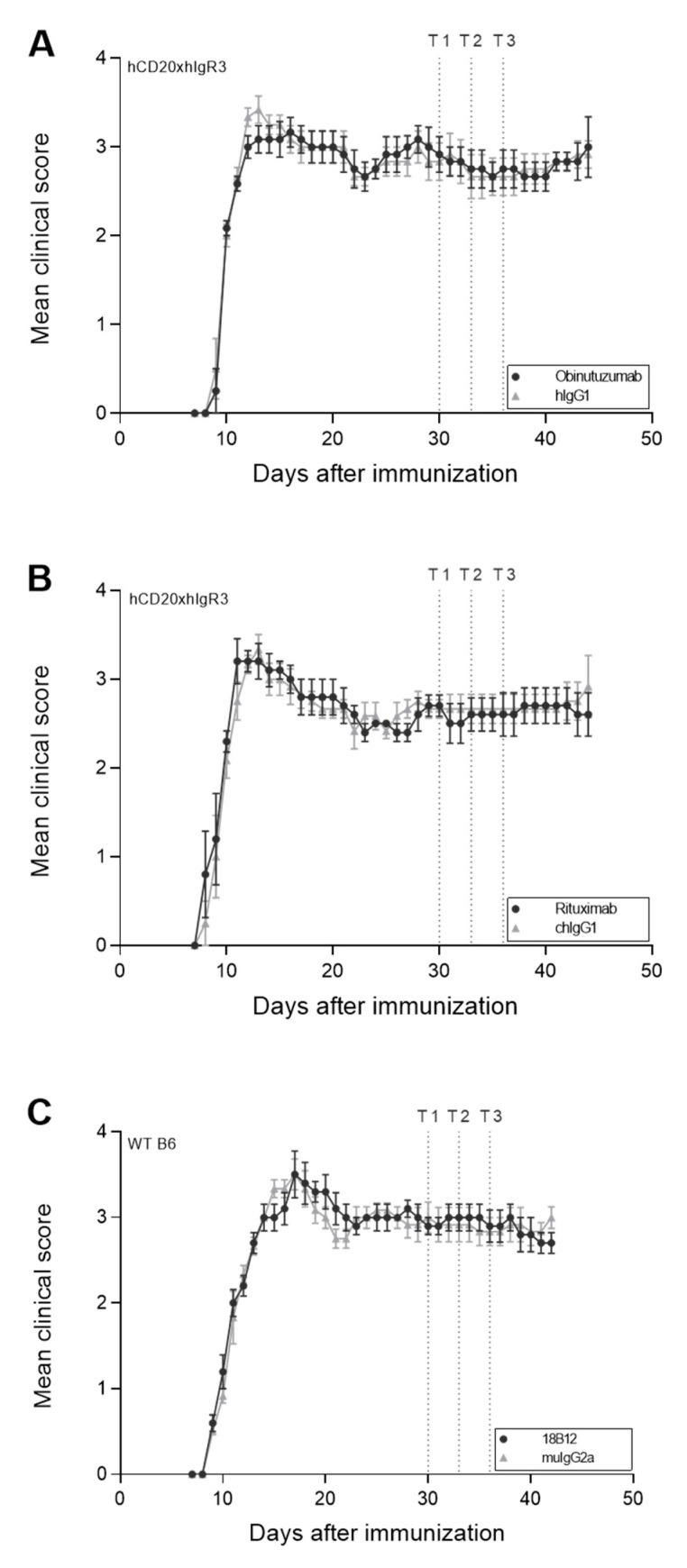
Figure 1.
Effect of anti-CD20 mAb treatment on clinical disease in hCD20xhIgR3 and WT B6 mice. EAE was assessed daily in all mice (n = 5–6 per group) and scores are shown as means ± standard error of the mean. Dotted lines mark the three time points (T1, day 19; T2, day 22; T3, day 25) of treatment with 5 mg/kg anti-CD20 mAb: (A) obinutuzumab, (B) rituximab, or (C) 18B12, or their respective isotype control antibodies (hIgG1, chIgG1, or muIgG2a). Note that treatment started 19 days after the mice had reached an EAE score of ≥2.5. Mice were scored using the standard EAE scale ranging from 0 to 5: 0, no signs of disease; 1, limp tail; 2, hindlimb weakness; 3, nearly complete or complete hindlimb paralysis; 4, complete hindlimb and partial forelimb paralysis; 5, moribund. The area under the curve (AUC) was calculated for each group. To evaluate whether there was a statistically significant difference in disease severity between the isotype control- and anti-CD20 mAb-treated groups the p-value of the AUCs was calculated using an unpaired t-test. No statistical significance was observed.