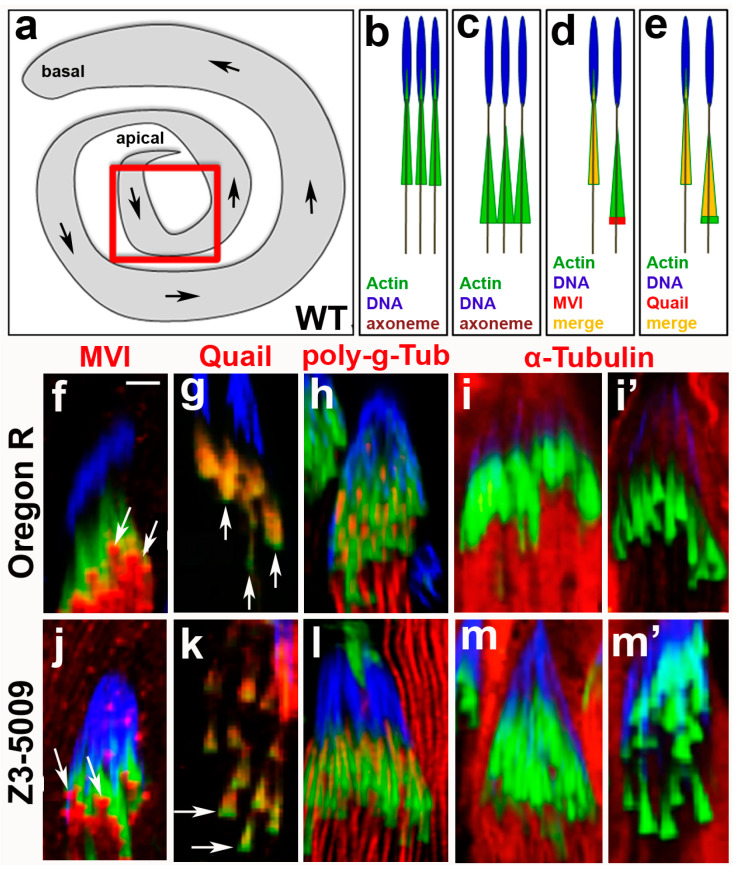
Figure 3.
Immunocytochemical characterization of Z3-5009 mutant. Schematic representations of: whole Drosophila testis (a), newly forming actin cones in association with spermatid nuclei (b), early actin cones that have just moved a little away from the nuclei (c), myosin VI localization in newly forming/early control (WT Oregon R) cones (d), and quail localization in newly forming/early control cones (e); red box in (a) shows area-of-interest for all of the images in this figure, arrows in (a) indicate the direction of actin cones movement during spermatid individualization. Immunofluorescence comparative analysis of localization of selected proteins in control (f–i’) and Z3-5009 (j–m’) actin cones; phalloidin staining, green; antibody of interest, red; merge, yellow; DAPI staining, blue. Myosin VI localizes to the front of conical actin cones in Z3-5009 mutant ((j), arrows), consistent with the same-stage Oregon R cones ((f), arrows). Actin-binding quail appears to proceed towards the rear domains of the control (g) and Z3-5009 (k) cones as seen by red staining, leaving more intense actin (green) areas in the front of the cones (arrows in (g,k)). Spermatid axonemes are highlighted by poly-glycylated tubulin (h,l) and axoneme organization is normal in the Z3-5009 mutant (l) if compared to Oregon R (h). Alpha-tubulin appears to degrade to a similar extent in the same-stage Oregon R ((i’) vs. (i)) and Z3-5009 ((m’) vs. (m)) actin cones. Bar 5 µm.