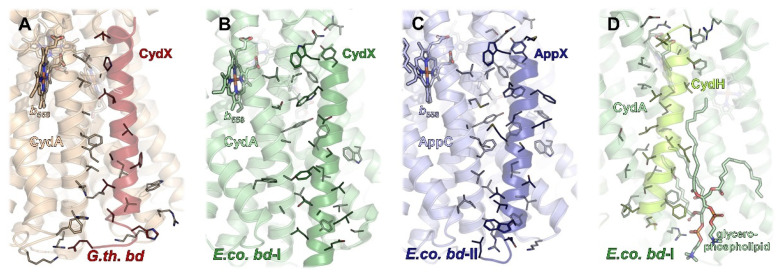
Figure 4.
Interaction interfaces of additional subunits with the bd core subunit CydA/AppC. (A–C) Subunit CydX/AppX (bold colours) binds to subunit CydA/AppC (light colours) in a largely conserved position close to heme b558, lateral to the Q binding site (not shown). Interactions are mainly driven by hydrophobic contacts. (A) Interaction patterns between CydX and CydA in G. thermodenitrificans bd. (B,C) CydX/AppX of E. coli bd-I and bd-II bind in a nearly identical manner to CydA/AppC, underlining the close homology of both bd oxidases. As compared to G. thermodenitrificans bd, the N-terminus (top) of CydX/AppX is tilted further away from heme b558. (D) Subunit CydH (limon), exclusive to E. coli bd-I, is found at the opposite side of CydA (light green) and appears to be further stabilised by a glycerophospholipid (shown as sticks). Again, mainly hydrophobic interactions contribute to binding.