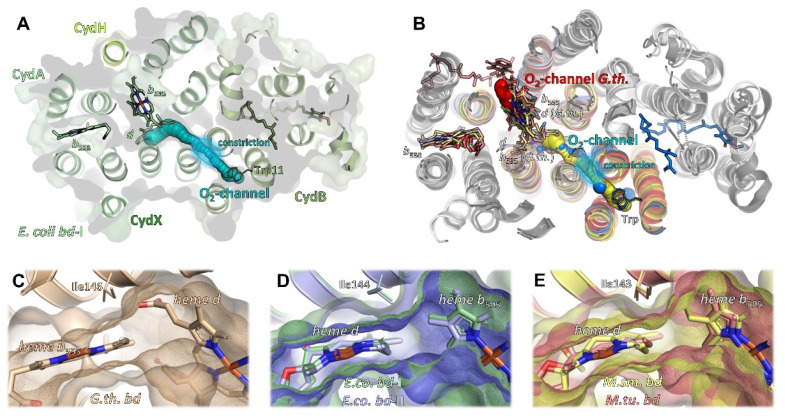
Figure 5.
Oxygen channels to the active site in bacterial bd oxidases. (A) Oxygen channel in E. coli bd-I (cyan) leading from Trp11 in CydB through the protein core to heme d. The channel features a constriction that is thought to serve as selectivity filter for linear molecules such as dioxygen. (B) Other bd oxidases feature an equivalent oxygen channel (E. coli bd-II in marine blue, M. smegmatis bd in yellow, M. tuberculosis bd in salmon) with a similar constriction. Only G. thermodenitrificans bd, as a consequence of the altered arrangement of the heme groups, features a very short channel (red) from the opposing side of the enzyme and leading directly to the active site heme d. (C–E) A conserved isoleucine residue (shown as thick lines) blocks diffusion of dioxygen between hemes b595 and d by perfect surface complementarity. Hemes are given as sticks, protein surfaces (smooth surfaces) and surfaces of hemes (meshes) are provided to illustrate the excellent surface match. (C) G. thermodenitrificans bd, (D) E. coli bd-I (pale green) and E. coli bd-II (slate blue), (E) M. smegmatis (pale yellow) and M. tuberculosis bd (salmon).