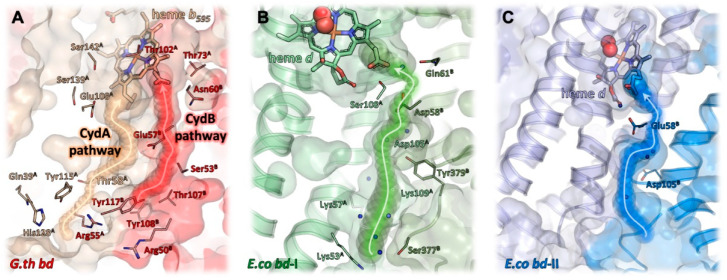
Figure 6.
Proton pathways in bacterial bd oxidases to the heme triangle. (A) G. thermodenitrificans features a pathway along the CydA/CydB interface, termed the CydB pathway (red). A second pathway running through CydA has been proposed as well, termed CydA pathway (light beige). (B) A pathway in E. coli bd-I (olive green), largely equivalent to the CydB pathway in G. thermodenitrificans, is lined by several hydrophilic amino acid sidechains (shown as thick lines) and directs water molecules (i.e., protons) to the propionate of the active site heme d. Numerous water molecules (blue spheres) have been found in that channel, highlighting its full accessibility. (C) E. coli bd-II features a comparable channel. But due to the much wider cavity protruding deeply into the protein core, the actual channel requires a only a glutamate and an aspartate sidechain (shown as sticks) to coordinate water molecules and guide them to the active site. Here again, the presence of water molecules illustrates the accessibility of solvent molecules.