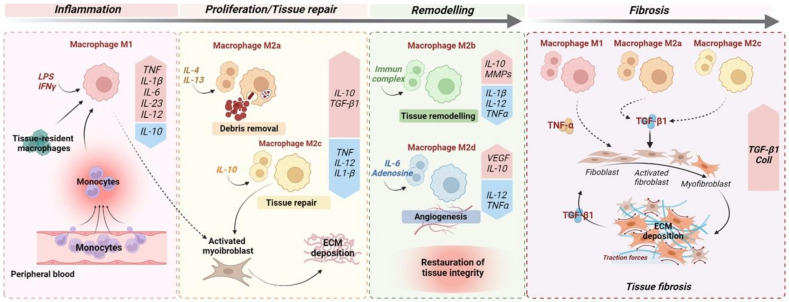
Figure 1.
Monocyte and macrophage polarisation during wound healing and fibrosis. The optimal wound healing process could be dived into three distinct phases: inflammation, proliferation, and remodelling. After an acute injury (left), the first phase of wound healing is predominated by pro-inflammatory signals, including lipopolysaccharides (LPSs), that stimulate pro-inflammatory M1 macrophages but also the recruitment upon injury of circulating monocytes, which develop into pro-inflammatory M1 macrophages. In turn, M1 macrophages secrete high concentrations of pro-inflammatory cytokines, such as TNF and IL1-β. In the meantime, IL-4 and IL-13 induce M2a macrophages that clear apoptotic cells. During the proliferative phase, in response to anti-inflammatory cytokines/mediators, including IL-4 and IL-13, and the efferocytosis of apoptotic cells ensured by M2a macrophages, macrophages undergo functional reprogramming toward a pro-restorative phenotype: M2c. These, later, by secreting TGF-β1, promote activation of myofibroblasts and extracellular matrix (ECM) deposition. During the last phase of wound healing, IL-10 is a key anti-inflammatory cytokine produced during the proliferative stage of repair that facilitates tissue remodelling by activating M2b macrophages which release metalloprotease matrix proteins (MMPs) to regulate ECM degradation. In addition, angiogenic response is promoted by M2d macrophage-releasing pro-angiogenic factors, including VEGF. Ultimately, the remodelling phase concludes with complete restoration of tissue. When chronic injuries occur (right), the persistence of M1, M2a, and M2c macrophage activation leads to fibrogenesis through the secretion of pro-inflammatory (TNF) and pro-fibrotic (TGF-β1) cytokines, resulting in sustained myofibroblast activation and leading to excessive ECM deposition.