Table 1.
Summary of the drugs products and active pharmaceutical ingredient (API) properties.
| Drug Product | Route of Administration |
Concentration | Excipients | Laboratory | API Structure | logP | pKa1 | pKa2 |
|---|---|---|---|---|---|---|---|---|
| Ketamine Chlorhydrate |
Intramuscular or intravenous | 50 mg mL−1 | Chlorobutanol, water for injection | Panpharma |
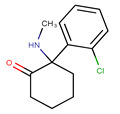 Ketamine |
2.65 | 7.16 | - |
| Midazolam Chlorhydrate | Intramuscular, intravenous or rectal | 5 mg mL−1 | NaOH, NaCl, HCl, water for injection | Mylan |
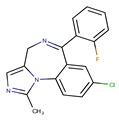 Midazolam |
3.33 | 3.48 | 6.57 |
| Clonidine chlorhydrate (Catapressan®) |
Intramuscularor intravenous | 0.15 mg mL−1 | NaCl, HCl, water for injection | Boehring |
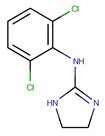 Clonidine |
2.49 | 8.16 | - |
| Sufentanil Citrate | Intravenous and epidural | 5 μg mL−1 | NaOH, NaCl, HCl, water for injection | Mylan |
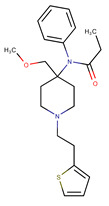 Sufentanil |
3.61 | 8.86 | - |
| Loxapine (Loxapac®) |
Intramuscular | 25 mg mL−1 | Polysorbate 80, Propylene glycol, HCl, water for injection | EISAI |
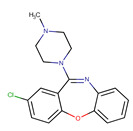 Loxapine |
3.46 | 1.02 | 7.18 |
