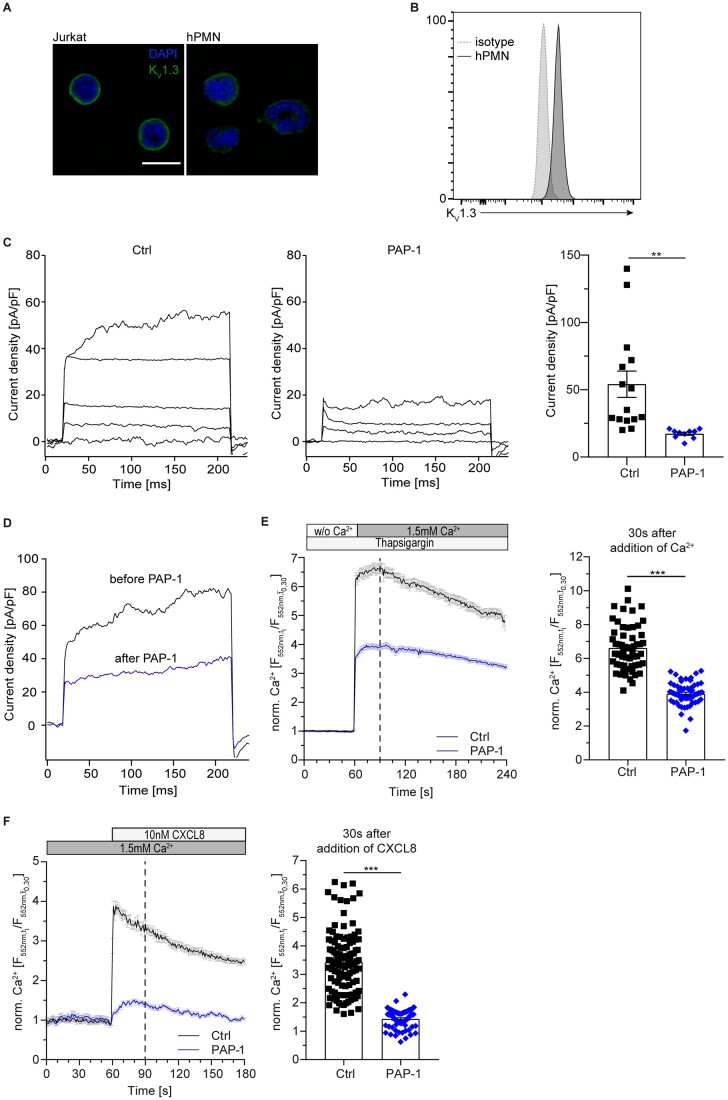
Figure 1.
KV1.3 regulates sustained calcium entry in human neutrophils. Surface localization of KV1.3 was determined by (A) confocal microscopy (representative images from n=3 independent experiments, scale bar =10 m) and (B) flow cytometry (representative histogram of n=5 independent experiments). (C) Patch-clamp was used to investigate functionality of KV1.3 in primary human neutrophils in absence and presence of KV1.3 inhibitor PAP-1 (10 nM) and quantified. Application of 13 consecutive 10 mV steps from −80 to +40 mV induced the development of voltage-activated potassium currents in control cells (representative traces no. 1, 4, 8, 13 of n=10–15 cells for each treatment). (D) K+ currents measured in primary human neutrophils in absence (black) and after direct application of PAP-1 (blue; 10 nM) after current activation with a single voltage-step to +40 mV (representative traces of n=4 cells). Isolated human neutrophils were loaded with Rhod-2 AM and subsequently pre-treated with PAP-1 (10 nM) or vehicle (Ctrl). Changes in [Ca2+]i were observed in Ca2+ free buffer and after the addition of 1.5 mM Ca2+. (E) CRAC channel dependent Ca2+ influx relative to baseline [mean(t0,t30)] in human neutrophils pre-treated with Thapsigargin was determined under static conditions and [Ca2+]i was quantified 30 s after addition of Ca2+ to the medium [n=57 (Ctrl) and 60 (PAP-1) cells from 3 independent experiments, unpaired Student’s t-test]. (F) Total Ca2+ flux was investigated after stimulation with CXCL8 (10 nM) under static conditions and total [Ca2+]i relative to baseline [mean(t0,t30)] was quantified 30 s after stimulation with CXCL8 [n=118 (Ctrl) and 69 (PAP-1) cells from 3 independent experiments, unpaired Student’s t-test]. ***P≤0.001, data in (A)–(D) are given as representative plots/images/traces, data in (C), (E), and (F) are shown as mean±SEM. Dotted lines in (E) and (F) represent the time points of quantification.